Impaired haemophilus influenzae type b transplacental antibody transmission and declining antibody avidity through the first year of life represent potential vulnerabilities for HIV-exposed but -uninfected infants
- PMID: 25298109
- PMCID: PMC4248779
- DOI: 10.1128/CVI.00356-14
Impaired haemophilus influenzae type b transplacental antibody transmission and declining antibody avidity through the first year of life represent potential vulnerabilities for HIV-exposed but -uninfected infants
Abstract
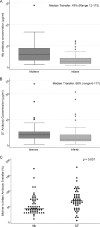
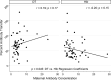
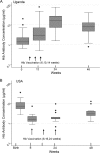
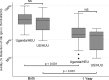
To determine whether immune function is impaired among HIV-exposed but -uninfected (HEU) infants born to HIV-infected mothers and to identify potential vulnerabilities to vaccine-preventable infection, we characterized the mother-to-infant placental transfer of Haemophilus influenzae type b-specific IgG (Hib-IgG) and its levels and avidity after vaccination in Ugandan HEU infants and in HIV-unexposed U.S. infants. Hib-IgG was measured by enzyme-linked immunosorbent assay in 57 Ugandan HIV-infected mothers prenatally and in their vaccinated HEU infants and 14 HIV-unexposed U.S. infants at birth and 12, 24, and 48 weeks of age. Antibody avidity at birth and 48 weeks of age was determined with 1 M ammonium thiocyanate. A median of 43% of maternal Hib-IgG was transferred to HEU infants. Although its level was lower in HEU infants than in U.S. infants at birth (P < 0.001), Hib-IgG was present at protective levels (>1.0 μg/ml) at birth in 90% of HEU infants and all U.S. infants. HEU infants had robust Hib-IgG responses to a primary vaccination. Although Hib-IgG levels declined from 24 to 48 weeks of age in HEU infants, they were higher than those in U.S. infants (P = 0.002). Antibody avidity, comparable at birth, declined by 48 weeks of age in both populations. Early vaccination of HEU infants may limit an initial vulnerability to Hib disease resulting from impaired transplacental antibody transfer. While initial Hib vaccine responses appeared adequate, the confluence of lower antibody avidity and declining Hib-IgG levels in HEU infants by 12 months support Hib booster vaccination at 1 year. Potential immunologic impairments of HEU infants should be considered in the development of vaccine platforms for populations with high maternal HIV prevalence.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
Safety and antibody persistence following Haemophilus influenzae type b conjugate or pneumococcal polysaccharide vaccines given before pregnancy in women of childbearing age and their infants.Pediatr Infect Dis J. 2001 Oct;20(10):931-40. doi: 10.1097/00006454-200110000-00005. Pediatr Infect Dis J. 2001. PMID: 11642626 Clinical Trial.
-
Antibody responses to vaccination among South African HIV-exposed and unexposed uninfected infants during the first 2 years of life.Clin Vaccine Immunol. 2013 Jan;20(1):33-8. doi: 10.1128/CVI.00557-12. Epub 2012 Oct 31. Clin Vaccine Immunol. 2013. PMID: 23114697 Free PMC article.
-
Maternal HIV infection and antibody responses against vaccine-preventable diseases in uninfected infants.JAMA. 2011 Feb 9;305(6):576-84. doi: 10.1001/jama.2011.100. JAMA. 2011. PMID: 21304083
-
Maternal immunization with Haemophilus influenzae type b vaccines in different populations.Vaccine. 2003 Jul 28;21(24):3455-9. doi: 10.1016/s0264-410x(03)00350-5. Vaccine. 2003. PMID: 12850359 Review.
-
Circulating innate and adaptive immunity against anti-Haemophilus influenzae type b.Hum Antibodies. 2019;27(3):201-212. doi: 10.3233/HAB-190373. Hum Antibodies. 2019. PMID: 30958343 Review.
Cited by
-
The Immune System of HIV-Exposed Uninfected Infants.Front Immunol. 2016 Sep 28;7:383. doi: 10.3389/fimmu.2016.00383. eCollection 2016. Front Immunol. 2016. PMID: 27733852 Free PMC article. Review.
-
HIV-Exposed Uninfected Infants in Zimbabwe: Insights into Health Outcomes in the Pre-Antiretroviral Therapy Era.Front Immunol. 2016 Jun 6;7:190. doi: 10.3389/fimmu.2016.00190. eCollection 2016. Front Immunol. 2016. PMID: 27375613 Free PMC article.
-
Maternal Human Immunodeficiency Virus-Associated Hypergammaglobulinemia Reduces Transplacental Transfer of Immunoglobulin G to Plasmodium falciparum Antigens in Cameroonian Neonates.Open Forum Infect Dis. 2016 May 10;3(2):ofw092. doi: 10.1093/ofid/ofw092. eCollection 2016 Apr. Open Forum Infect Dis. 2016. PMID: 28487863 Free PMC article.
-
Crohn's Patients and Healthy Infants Share Immunodominant B Cell Response to Commensal Flagellin Peptide Epitopes.Gastroenterology. 2024 Dec;167(7):1415-1428. doi: 10.1053/j.gastro.2024.08.015. Epub 2024 Aug 21. Gastroenterology. 2024. PMID: 39173722
-
Which New Health Technologies Do We Need to Achieve an End to HIV/AIDS?PLoS Biol. 2016 Mar 2;14(3):e1002372. doi: 10.1371/journal.pbio.1002372. eCollection 2016 Mar. PLoS Biol. 2016. PMID: 26933962 Free PMC article. Review.
References
-
- St. Geme J. 2008. Haemophilus influenzae, p 892–897 In Long S, Pickering L, Prober C. (ed), Principles and practice of pediatric infectious diseases, 3rd ed. Elsevier, Inc., Philadelphia, PA.
-
- Peltola H. 2000. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clin. Microbiol. Rev. 13:302–317. 10.1128/CMR.13.2.302-317.2000. - DOI - PMC - PubMed
-
- Watt JP, Wolfson LJ, O'Brien KL, Henkle E, Deloria-Knoll M, McCall N, Lee E, Levine OS, Hajjeh R, Mulholland K, Cherian T. 2009. Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: global estimates. Lancet 374:903–911. 10.1016/S0140-6736(09)61203-4. - DOI - PubMed
-
- von Gottberg A, Cohen C, Whitelaw A, Chhagan M, Flannery B, Cohen AL, de Gouveia L, Plessis MD, Madhi SA, Klugman KP, Group for Enteric, Respiratory, Meningeal Disease Surveillance in South Africa (GERMS-SA) 2012. Invasive disease due to Haemophilus influenzae serotype b ten years after routine vaccination, South Africa, 2003-2009. Vaccine 30:565–571. 10.1016/j.vaccine.2011.11.066. - DOI - PubMed
-
- Lee EH, Lewis RF, Makumbi I, Kekitiinwa A, Ediamu TD, Bazibu M, Braka F, Flannery B, Zuber PL, Feikin DR. 2008. Haemophilus influenzae type b conjugate vaccine is highly effective in the Ugandan routine immunization program: a case-control study. Trop. Med. Int. Health 13:495–502. 10.1111/j.1365-3156.2008.02027.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

