Serelaxin-mediated signal transduction in human vascular cells: bell-shaped concentration-response curves reflect differential coupling to G proteins
- PMID: 25297987
- PMCID: PMC4314191
- DOI: 10.1111/bph.12964
Serelaxin-mediated signal transduction in human vascular cells: bell-shaped concentration-response curves reflect differential coupling to G proteins
Abstract
Background and purpose: In a recently conducted phase III clinical trial, RELAX-AHF, serelaxin infusion over 48 h improved short- and long-term clinical outcomes in patients with acute heart failure. In this study we used human primary cells from the umbilical vasculature to better understand the signalling mechanisms activated by serelaxin.
Experimental approach: We examined the acute effects of serelaxin on signal transduction mechanisms in primary human umbilical vascular cells and its chronic actions on markers of cardiovascular function and disease.
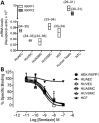
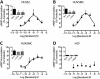
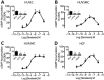
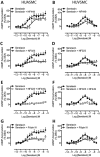
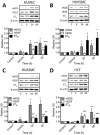
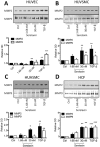
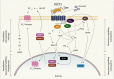
Key results: The RXFP1 receptor, the cognate serelaxin receptor, was expressed at the cell surface in HUVECs and human umbilical vein smooth muscle cells (HUVSMCs), human umbilical artery smooth muscle cells (HUASMCs) and human cardiac fibroblasts (HCFs), but not human umbilical artery endothelial cells. In HUVECs and HUVSMCs, serelaxin increased cAMP, cGMP accumulation and pERK1/2, and the concentration-response curves (CRCs) were bell-shaped. Similar bell-shaped CRCs for cGMP and pERK1/2 were observed in HCFs, whereas in HUASMCs, serelaxin increased cAMP, cGMP and pERK1/2 with sigmoidal CRCs. Gαi/o and lipid raft disruption, but not Gαs inhibition, altered the serelaxin CRC for cAMP and cGMP accumulation in HUVSMC but not HUASMC. Longer term serelaxin exposure increased the expression of neuronal NOS, VEGF, ETβ receptors and MMPs (gelatinases) in RXFP1 receptor-expressing cells.
Conclusions and implications: Serelaxin caused acute and chronic changes in human umbilical vascular cells that were cell background dependent. Bell-shaped CRCs that were observed only in venous cells and fibroblasts involved Gαi/o located within membrane lipid rafts.
© 2014 The British Pharmacological Society.
Figures

Similar articles
-
Enhanced serelaxin signalling in co-cultures of human primary endothelial and smooth muscle cells.Br J Pharmacol. 2016 Feb;173(3):484-96. doi: 10.1111/bph.13371. Epub 2016 Jan 15. Br J Pharmacol. 2016. PMID: 26493539 Free PMC article.
-
Localization of relaxin receptors in arteries and veins, and region-specific increases in compliance and bradykinin-mediated relaxation after in vivo serelaxin treatment.FASEB J. 2014 Jan;28(1):275-87. doi: 10.1096/fj.13-233429. Epub 2013 Sep 13. FASEB J. 2014. PMID: 24036884 Free PMC article.
-
Serelaxin alleviates cardiac fibrosis through inhibiting endothelial-to-mesenchymal transition via RXFP1.Theranostics. 2020 Mar 4;10(9):3905-3924. doi: 10.7150/thno.38640. eCollection 2020. Theranostics. 2020. PMID: 32226528 Free PMC article.
-
Serelaxin in acute heart failure: Most recent update on clinical and preclinical evidence.Cardiovasc Ther. 2017 Feb;35(1):55-63. doi: 10.1111/1755-5922.12231. Cardiovasc Ther. 2017. PMID: 27727514 Review.
-
Serelaxin: a novel therapy for acute heart failure with a range of hemodynamic and non-hemodynamic actions.Am J Cardiovasc Drugs. 2014 Aug;14(4):275-85. doi: 10.1007/s40256-014-0069-0. Am J Cardiovasc Drugs. 2014. PMID: 24590581 Free PMC article. Review.
Cited by
-
Absent or Excessive Corpus Luteum Number Is Associated With Altered Maternal Vascular Health in Early Pregnancy.Hypertension. 2019 Mar;73(3):680-690. doi: 10.1161/HYPERTENSIONAHA.118.12046. Hypertension. 2019. PMID: 30636549 Free PMC article.
-
The actions of relaxin on the human cardiovascular system.Br J Pharmacol. 2017 May;174(10):933-949. doi: 10.1111/bph.13523. Epub 2016 Jul 11. Br J Pharmacol. 2017. PMID: 27239943 Free PMC article. Review.
-
Serelaxin Treatment Reduces Oxidative Stress and Increases Aldehyde Dehydrogenase-2 to Attenuate Nitrate Tolerance.Front Pharmacol. 2017 Mar 21;8:141. doi: 10.3389/fphar.2017.00141. eCollection 2017. Front Pharmacol. 2017. PMID: 28377719 Free PMC article.
-
ML290 is a biased allosteric agonist at the relaxin receptor RXFP1.Sci Rep. 2017 Jun 7;7(1):2968. doi: 10.1038/s41598-017-02916-5. Sci Rep. 2017. PMID: 28592882 Free PMC article.
-
G protein βγ subunits inhibit TRPM3 ion channels in sensory neurons.Elife. 2017 Aug 15;6:e26138. doi: 10.7554/eLife.26138. Elife. 2017. PMID: 28826490 Free PMC article.
References
-
- Baker JG, Hill SJ. A comparison of the antagonist affinities for the Gi- and Gs-coupled states of the human adenosine A1-receptor. J Pharmacol Exp Ther. 2006;320:218–228. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

