Modest influence of FRET chromophores on the properties of unfolded proteins
- PMID: 25296318
- PMCID: PMC4190605
- DOI: 10.1016/j.bpj.2014.07.071
Modest influence of FRET chromophores on the properties of unfolded proteins
Abstract
Single-molecule Förster resonance energy transfer (FRET) experiments are often used to study the properties of unfolded and intrinsically disordered proteins. Because of their large extinction coefficients and quantum yields, synthetic heteroaromatic chromophores covalently linked to the protein are often used as donor and acceptor fluorophores. A key issue in the interpretation of such experiments is the extent to which the properties of the unfolded chain may be affected by the presence of these chromophores. In this article, we investigate this question using all-atom explicit solvent replica exchange molecular dynamics simulations of three different unfolded or intrinsically disordered proteins. We find that the secondary structure and long-range contacts are largely the same in the presence or absence of the fluorophores, and that the dimensions of the chain with and without chromophores are similar. This suggests that, at least in the cases studied, extrinsic fluorophores have little effect on the structural properties of unfolded or disordered proteins. We also find that the critical FRET orientational factor κ(2), has an average value and equilibrium distribution very close to that expected for isotropic orientations, which supports one of the assumptions frequently made when interpreting FRET efficiency in terms of distances.
Copyright © 2014 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
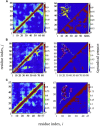
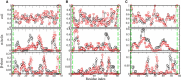
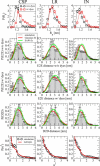
Similar articles
-
Probing the Action of Chemical Denaturant on an Intrinsically Disordered Protein by Simulation and Experiment.J Am Chem Soc. 2016 Sep 14;138(36):11702-13. doi: 10.1021/jacs.6b05443. Epub 2016 Sep 1. J Am Chem Soc. 2016. PMID: 27583687 Free PMC article.
-
Perspective: Chain dynamics of unfolded and intrinsically disordered proteins from nanosecond fluorescence correlation spectroscopy combined with single-molecule FRET.J Chem Phys. 2018 Jul 7;149(1):010901. doi: 10.1063/1.5037683. J Chem Phys. 2018. PMID: 29981536
-
Accurate Transfer Efficiencies, Distance Distributions, and Ensembles of Unfolded and Intrinsically Disordered Proteins From Single-Molecule FRET.Methods Enzymol. 2018;611:287-325. doi: 10.1016/bs.mie.2018.09.030. Epub 2018 Nov 16. Methods Enzymol. 2018. PMID: 30471690 Free PMC article.
-
Understanding disordered and unfolded proteins using single-molecule FRET and polymer theory.Methods Appl Fluoresc. 2016 Nov 17;4(4):042003. doi: 10.1088/2050-6120/4/4/042003. Methods Appl Fluoresc. 2016. PMID: 28192291 Review.
-
Single-Molecule FRET Spectroscopy and the Polymer Physics of Unfolded and Intrinsically Disordered Proteins.Annu Rev Biophys. 2016 Jul 5;45:207-31. doi: 10.1146/annurev-biophys-062215-010915. Epub 2016 May 2. Annu Rev Biophys. 2016. PMID: 27145874 Review.
Cited by
-
Probing the Action of Chemical Denaturant on an Intrinsically Disordered Protein by Simulation and Experiment.J Am Chem Soc. 2016 Sep 14;138(36):11702-13. doi: 10.1021/jacs.6b05443. Epub 2016 Sep 1. J Am Chem Soc. 2016. PMID: 27583687 Free PMC article.
-
Sequence- and Temperature-Dependent Properties of Unfolded and Disordered Proteins from Atomistic Simulations.J Phys Chem B. 2015 Nov 19;119(46):14622-30. doi: 10.1021/acs.jpcb.5b08619. Epub 2015 Nov 10. J Phys Chem B. 2015. PMID: 26498157 Free PMC article.
-
Finding Our Way in the Dark Proteome.J Am Chem Soc. 2016 Aug 10;138(31):9730-42. doi: 10.1021/jacs.6b06543. Epub 2016 Jul 19. J Am Chem Soc. 2016. PMID: 27387657 Free PMC article.
-
Effects of Sequence Composition, Patterning and Hydrodynamics on the Conformation and Dynamics of Intrinsically Disordered Proteins.Int J Mol Sci. 2023 Jan 11;24(2):1444. doi: 10.3390/ijms24021444. Int J Mol Sci. 2023. PMID: 36674958 Free PMC article.
-
Quantitative interpretation of FRET experiments via molecular simulation: force field and validation.Biophys J. 2015 Jun 2;108(11):2721-31. doi: 10.1016/j.bpj.2015.04.038. Biophys J. 2015. PMID: 26039173 Free PMC article.
References
-
- Förster T. Intermolecular energy migration and fluorescence [Zwischenmolekulare energiewanderung und fluoreszenz] Ann. Phys. 1948;2:55–75.
-
- Förster T. Delocalized excitation and excitation transfer. In: Sinanoglu O., editor. Modern Quantum Chemistry: Istanbul Lectures. Part III, Action of Light and Organic Crystals. Academic Press; New York: 1965.
-
- Kuzmenkina E.V., Heyes C.D., Nienhaus G.U. Single-molecule FRET study of denaturant induced unfolding of RNase H. J. Mol. Biol. 2006;357:313–324. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

