Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients
- PMID: 25286015
- PMCID: PMC4266079
- DOI: 10.3109/21678421.2014.959024
Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients
Abstract
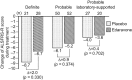
Our objective was to confirm the efficacy and safety of edaravone in amyotrophic lateral sclerosis (ALS) patients. We conducted a 36-week confirmatory study, consisting of 12-week pre-observation period followed by 24-week treatment period. Patients received placebo or edaravone i.v. infusion over 60 min for the first 14 days in cycle 1, and for 10 of the first 14 days during cycles 2 to 6. The efficacy primary endpoint was changed in the revised ALS functional rating scale (ALSFRS-R) scores during the 24-week treatment. Patients were treated with placebo (n = 104) and edaravone (n = 102). Changes in ALSFRS-R during the 24-week treatment were -6.35 ± 0.84 in the placebo group (n = 99) and -5.70 ± 0.85 in the edaravone group (n = 100), with a difference of 0.65 ± 0.78 (p = 0.411). Adverse events amounted to 88.5% (92/104) in the placebo group and 89.2% (91/102) in the edaravone group. In conclusion, the reduction of ALSFRS-R was smaller in the edaravone group than in the placebo group, but efficacy of edaravone for treatment of ALS was not demonstrated. Levels and frequencies of reported adverse events were similar in the two groups.
Keywords: ALSFRS-R; Amyotrophic lateral sclerosis; edaravone; placebo; randomized trial.
Figures
Similar articles
-
Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial.Lancet Neurol. 2017 Jul;16(7):505-512. doi: 10.1016/S1474-4422(17)30115-1. Epub 2017 May 15. Lancet Neurol. 2017. PMID: 28522181 Clinical Trial.
-
Exploratory double-blind, parallel-group, placebo-controlled study of edaravone (MCI-186) in amyotrophic lateral sclerosis (Japan ALS severity classification: Grade 3, requiring assistance for eating, excretion or ambulation).Amyotroph Lateral Scler Frontotemporal Degener. 2017 Oct;18(sup1):40-48. doi: 10.1080/21678421.2017.1361441. Amyotroph Lateral Scler Frontotemporal Degener. 2017. PMID: 28872915 Clinical Trial.
-
Exploratory double-blind, parallel-group, placebo-controlled extension study of edaravone (MCI-186) in amyotrophic lateral sclerosis.Amyotroph Lateral Scler Frontotemporal Degener. 2017 Oct;18(sup1):20-31. doi: 10.1080/21678421.2017.1362000. Amyotroph Lateral Scler Frontotemporal Degener. 2017. PMID: 28872918 Clinical Trial.
-
Edaravone and its clinical development for amyotrophic lateral sclerosis.Amyotroph Lateral Scler Frontotemporal Degener. 2017 Oct;18(sup1):5-10. doi: 10.1080/21678421.2017.1353101. Amyotroph Lateral Scler Frontotemporal Degener. 2017. PMID: 28872907 Review.
-
Clinical efficacy of edaravone for the treatment of amyotrophic lateral sclerosis.Expert Opin Pharmacother. 2017 May;18(7):735-738. doi: 10.1080/14656566.2017.1319937. Expert Opin Pharmacother. 2017. PMID: 28406335 Review.
Cited by
-
Superoxide dismutase and neurological disorders.IBRO Neurosci Rep. 2024 Jan 23;16:373-394. doi: 10.1016/j.ibneur.2023.11.007. eCollection 2024 Jun. IBRO Neurosci Rep. 2024. PMID: 39007083 Free PMC article. Review.
-
Mouse Nerve Growth Factor Injection and Progression Rate in Patients With Amyotrophic Lateral Sclerosis: An Observational Study.Front Neurol. 2022 Feb 17;13:829569. doi: 10.3389/fneur.2022.829569. eCollection 2022. Front Neurol. 2022. PMID: 35250834 Free PMC article.
-
An Exploratory Trial of EPI-589 in Amyotrophic Lateral Sclerosis (EPIC-ALS): Protocol for a Multicenter, Open-Labeled, 24-Week, Single-Group Study.JMIR Res Protoc. 2023 Jan 30;12:e42032. doi: 10.2196/42032. JMIR Res Protoc. 2023. PMID: 36716091 Free PMC article.
-
Amplifying the Heat Shock Response Ameliorates ALS and FTD Pathology in Mouse and Human Models.Mol Neurobiol. 2023 Dec;60(12):6896-6915. doi: 10.1007/s12035-023-03509-2. Epub 2023 Jul 29. Mol Neurobiol. 2023. PMID: 37516663 Free PMC article.
-
An overview on recent in vivo biological application of cerium oxide nanoparticles.Asian J Pharm Sci. 2020 Sep;15(5):558-575. doi: 10.1016/j.ajps.2019.10.005. Epub 2019 Nov 27. Asian J Pharm Sci. 2020. PMID: 33193860 Free PMC article. Review.
References
-
- Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344:1688–700. - PubMed
-
- Beckman JS, Carson M, Smith CD, Koppenol W. ALS, SOD and peroxynitrite. Nature. 1993;364:584. - PubMed
-
- Ferrante RJ, Shinobu LA, Schulz JB, Matthews RT, Thomas CE, Kowall NW, et al. Increased 3-nitrotyrosine and oxidative damage in mice with a human Cu/Zn superoxide disumutase mutation. Ann Neurol. 1997;42:326–34. - PubMed
-
- Beal MF, Ferrante RJ, Browne SE, Matthews RT, Kowall NW, Brown RH., Jr Increased 3-nitrotyrosine in both sporadic and familial amyotrophic lateral sclerosis. Ann Neurol. 1997;42:644–54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous