A regimen combining the Wee1 inhibitor AZD1775 with HDAC inhibitors targets human acute myeloid leukemia cells harboring various genetic mutations
- PMID: 25283841
- PMCID: PMC4387110
- DOI: 10.1038/leu.2014.296
A regimen combining the Wee1 inhibitor AZD1775 with HDAC inhibitors targets human acute myeloid leukemia cells harboring various genetic mutations
Abstract
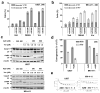
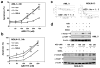
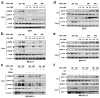
AZD1775 targets the cell cycle checkpoint kinase Wee1 and potentiates genotoxic agent cytotoxicity through p53-dependent or -independent mechanisms. Here, we report that AZD1775 interacted synergistically with histone deacetylase inhibitors (HDACIs, for example, Vorinostat), which interrupt the DNA damage response, to kill p53-wild type (wt) or -deficient as well as FLT3-ITD leukemia cells in association with pronounced Wee1 inhibition and diminished cdc2/Cdk1 Y15 phosphorylation. Similarly, Wee1 shRNA knockdown significantly sensitized cells to HDACIs. Although AZD1775 induced Chk1 activation, reflected by markedly increased Chk1 S296/S317/S345 phosphorylation leading to inhibitory T14 phosphorylation of cdc2/Cdk1, these compensatory responses were sharply abrogated by HDACIs. This was accompanied by premature mitotic entry, multiple mitotic abnormalities and accumulation of early S-phase cells displaying increased newly replicated DNA, culminating in robust DNA damage and apoptosis. The regimen was active against patient-derived acute myelogenous leukemia (AML) cells harboring either wt or mutant p53 and various next-generation sequencing-defined mutations. Primitive CD34(+)/CD123(+)/CD38(-) populations enriched for leukemia-initiating progenitors, but not normal CD34(+) hematopoietic cells, were highly susceptible to this regimen. Finally, combining AZD1775 with Vorinostat in AML murine xenografts significantly reduced tumor burden and prolonged animal survival. A strategy combining Wee1 with HDACI inhibition warrants further investigation in AML with poor prognostic genetic aberrations.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures


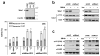


Similar articles
-
The NAE inhibitor pevonedistat interacts with the HDAC inhibitor belinostat to target AML cells by disrupting the DDR.Blood. 2016 May 5;127(18):2219-30. doi: 10.1182/blood-2015-06-653717. Epub 2016 Feb 5. Blood. 2016. PMID: 26851293 Free PMC article.
-
The novel Chk1 inhibitor MK-8776 sensitizes human leukemia cells to HDAC inhibitors by targeting the intra-S checkpoint and DNA replication and repair.Mol Cancer Ther. 2013 Jun;12(6):878-89. doi: 10.1158/1535-7163.MCT-12-0902. Epub 2013 Mar 27. Mol Cancer Ther. 2013. PMID: 23536721 Free PMC article.
-
Replication Stress Leading to Apoptosis within the S-phase Contributes to Synergism between Vorinostat and AZD1775 in HNSCC Harboring High-Risk TP53 Mutation.Clin Cancer Res. 2017 Nov 1;23(21):6541-6554. doi: 10.1158/1078-0432.CCR-17-0947. Epub 2017 Aug 8. Clin Cancer Res. 2017. PMID: 28790110 Free PMC article.
-
Strategic development of AZD1775, a Wee1 kinase inhibitor, for cancer therapy.Expert Opin Investig Drugs. 2018 Sep;27(9):741-751. doi: 10.1080/13543784.2018.1511700. Epub 2018 Aug 21. Expert Opin Investig Drugs. 2018. PMID: 30102076 Review.
-
Dysfunctional diversity of p53 proteins in adult acute myeloid leukemia: projections on diagnostic workup and therapy.Blood. 2017 Aug 10;130(6):699-712. doi: 10.1182/blood-2017-02-763086. Epub 2017 Jun 12. Blood. 2017. PMID: 28607134 Free PMC article. Review.
Cited by
-
Targeting the DNA damage response in hematological malignancies.Front Oncol. 2024 Jan 29;14:1307839. doi: 10.3389/fonc.2024.1307839. eCollection 2024. Front Oncol. 2024. PMID: 38347838 Free PMC article. Review.
-
Bioinformatics Analysis Identifies EPAS1 as a Novel Prognostic Marker Correlated with Immune Infiltration in Acute Myeloid Leukemia.Dis Markers. 2023 Apr 17;2023:6072782. doi: 10.1155/2023/6072782. eCollection 2023. Dis Markers. 2023. PMID: 37124944 Free PMC article.
-
HDAC11 mediates the ubiquitin-dependent degradation of p53 and inhibits the anti-leukemia effect of PD0166285.Med Oncol. 2023 Oct 7;40(11):325. doi: 10.1007/s12032-023-02196-2. Med Oncol. 2023. PMID: 37805625
-
Synergistic anti-leukemic interactions between panobinostat and MK-1775 in acute myeloid leukemia ex vivo.Cancer Biol Ther. 2015;16(12):1784-93. doi: 10.1080/15384047.2015.1095406. Cancer Biol Ther. 2015. PMID: 26529495 Free PMC article.
-
WEE1 is a validated target of the microRNA miR-17-92 cluster in leukemia.Cancer Genet. 2015 May;208(5):279-87. doi: 10.1016/j.cancergen.2015.01.001. Epub 2015 Jan 20. Cancer Genet. 2015. PMID: 25732734 Free PMC article.
References
-
- Roboz GJ, Rosenblat T, Arellano M, Gobbi M, Altman JK, Montesinos P, et al. International Randomized Phase III Study of Elacytarabine Versus Investigator Choice in Patients With Relapsed/Refractory Acute Myeloid Leukemia. J Clin Oncol. 2014 Jun 20;32(18):1919–26. - PubMed
-
- Grant S, Dai Y. Histone deacetylase inhibitors and rational combination therapies. Adv Cancer Res. 2012;116:199–237. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

