Anti-JC virus antibody levels in serum or plasma further define risk of natalizumab-associated progressive multifocal leukoencephalopathy
- PMID: 25273271
- PMCID: PMC4282070
- DOI: 10.1002/ana.24286
Anti-JC virus antibody levels in serum or plasma further define risk of natalizumab-associated progressive multifocal leukoencephalopathy
Abstract
Objective: The increased risk of progressive multifocal leukoencephalopathy (PML) with natalizumab treatment is associated with the presence of anti-JC virus (JCV) antibodies. We analyzed whether anti-JCV antibody levels, measured as index, may further define PML risk in seropositive patients.
Methods: The association between serum or plasma anti-JCV antibody levels and PML risk was examined in anti-JCV antibody-positive multiple sclerosis (MS) patients from natalizumab clinical studies and postmarketing sources. For PML and non-PML patients, the probabilities of having an index below and above a range of anti-JCV antibody index thresholds were calculated using all available data and applied to the PML risk stratification algorithm. Longitudinal stability of anti-JCV antibody index was also evaluated.
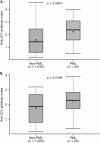
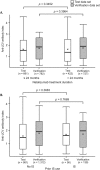
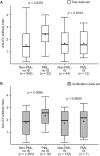
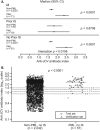
Results: Anti-JCV antibody index data were available for serum/plasma samples collected >6 months prior to PML diagnosis from 71 natalizumab-treated PML patients and 2,522 non-PML anti-JCV antibody-positive patients. In patients with no prior immunosuppressant use, anti-JCV antibody index distribution was significantly higher in PML patients than in non-PML patients (p < 0.0001). Among patients who were anti-JCV antibody negative at baseline in the AFFIRM and STRATIFY-1 trials, 97% remained consistently negative or below an index threshold of 1.5 over 18 months. Retrospective analyses of pre-PML samples collected longitudinally from PML patients displayed sustained higher anti-JCV antibody index over time.
Interpretation: Anti-JCV antibody levels in serum/plasma, measured as index, may differentiate PML risk in anti-JCV antibody-positive MS patients with no prior immunosuppressant use. Continued evaluation of anti-JCV antibody index and PML risk is warranted.
© 2014 Biogen Idec. Annals of Neurology published by Wiley Periodicals, Inc. on behalf of American Neurological Association.
Figures

Similar articles
-
Risk of natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: a retrospective analysis of data from four clinical studies.Lancet Neurol. 2017 Nov;16(11):925-933. doi: 10.1016/S1474-4422(17)30282-X. Epub 2017 Sep 29. Lancet Neurol. 2017. PMID: 28969984
-
The effect of plasma exchange on serum anti-JC virus antibodies.Mult Scler. 2013 Jun;19(7):912-9. doi: 10.1177/1352458512467502. Epub 2012 Dec 11. Mult Scler. 2013. PMID: 23232602 Clinical Trial.
-
Anti-JC virus antibodies in a large German natalizumab-treated multiple sclerosis cohort.Neurology. 2012 May 29;78(22):1736-42. doi: 10.1212/WNL.0b013e3182583022. Epub 2012 May 16. Neurology. 2012. PMID: 22592369
-
Re-evaluating the incidence of natalizumab-associated progressive multifocal leukoencephalopathy.Mult Scler Relat Disord. 2016 Jul;8:145-50. doi: 10.1016/j.msard.2016.03.005. Epub 2016 Apr 1. Mult Scler Relat Disord. 2016. PMID: 27456891 Review.
-
Natalizumab: risk stratification of individual patients with multiple sclerosis.CNS Drugs. 2014 Jul;28(7):641-8. doi: 10.1007/s40263-014-0168-0. CNS Drugs. 2014. PMID: 24942634 Review.
Cited by
-
Benefit-Risk of Therapies for Relapsing-Remitting Multiple Sclerosis: Testing the Number Needed to Treat to Benefit (NNTB), Number Needed to Treat to Harm (NNTH) and the Likelihood to be Helped or Harmed (LHH): A Systematic Review and Meta-Analysis.CNS Drugs. 2016 Oct;30(10):909-29. doi: 10.1007/s40263-016-0377-9. CNS Drugs. 2016. PMID: 27538416 Review.
-
Detection of cases of progressive multifocal leukoencephalopathy associated with new biologicals and targeted cancer therapies from the FDA's adverse event reporting system.Expert Opin Drug Saf. 2016 Aug;15(8):1003-11. doi: 10.1080/14740338.2016.1198775. Epub 2016 Jun 20. Expert Opin Drug Saf. 2016. PMID: 27268272 Free PMC article.
-
Treatment-Related Progressive Multifocal Leukoencephalopathy in Multiple Sclerosis: A Comprehensive Review of Current Evidence and Future Needs.Drug Saf. 2016 Dec;39(12):1163-1174. doi: 10.1007/s40264-016-0461-6. Drug Saf. 2016. PMID: 27696299 Review.
-
Natalizumab-treated patients at high risk for PML persistently excrete JC polyomavirus.J Neurovirol. 2016 Dec;22(6):871-875. doi: 10.1007/s13365-016-0449-0. Epub 2016 May 19. J Neurovirol. 2016. PMID: 27198748 Free PMC article.
-
Training physicians in providing complex information to patients with multiple sclerosis: a randomised controlled trial.BMJ Open. 2022 Mar 15;12(3):e049817. doi: 10.1136/bmjopen-2021-049817. BMJ Open. 2022. PMID: 35292486 Free PMC article. Clinical Trial.
References
-
- Polman CH, O'Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. - PubMed
-
- Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366:1870–1880. - PubMed
-
- Biogen Idec. Medical information website. Available at: https://medinfo.biogenidec.com/medinfo. Accessed September 2012 to August 30, 2014.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

