Leukocytes as carriers for targeted cancer drug delivery
- PMID: 25270379
- PMCID: PMC5658012
- DOI: 10.1517/17425247.2015.966684
Leukocytes as carriers for targeted cancer drug delivery
Abstract
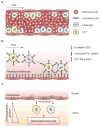
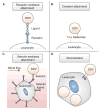
Introduction: Metastasis contributes to over 90% of cancer-related deaths. Numerous nanoparticle platforms have been developed to target and treat cancer, yet efficient delivery of these systems to the appropriate site remains challenging. Leukocytes, which share similarities to tumor cells in terms of their transport and migration through the body, are well suited to serve as carriers of drug delivery systems to target cancer sites.
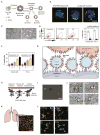
Areas covered: This review focuses on the use and functionalization of leukocytes for therapeutic targeting of metastatic cancer. Tumor cell and leukocyte extravasation, margination in the bloodstream, and migration into soft tissue are discussed, along with the potential to exploit these functional similarities to effectively deliver drugs. Current nanoparticle-based drug formulations for the treatment of cancer are reviewed, along with methods to functionalize delivery vehicles to leukocytes, either on the surface and/or within the cell. Recent progress in this area, both in vitro and in vivo, is also discussed, with a particular emphasis on targeting cancer cells in the bloodstream as a means to interrupt the metastatic process.
Expert opinion: Leukocytes interact with cancer cells both in the bloodstream and at the site of solid tumors. These interactions can be utilized to effectively deliver drugs to targeted areas, which can reduce both the amount of drug required and various nonspecific cytotoxic effects within the body. If drug delivery vehicle functionalization does not interfere with leukocyte function, this approach may be utilized to neutralize tumor cells in the bloodstream to prevent the formation of new metastases, and also to deliver drugs to metastatic sites within tissues.
Keywords: cancer; leukocyte; metastasis; nanomedicine.
Conflict of interest statement
The authors were supported by the Cornell Center on the Microenvironment and Metastasis through Award Number U54CA143876 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures

Similar articles
-
Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy.Eur J Pharm Biopharm. 2015 Jun;93:52-79. doi: 10.1016/j.ejpb.2015.03.018. Epub 2015 Mar 23. Eur J Pharm Biopharm. 2015. PMID: 25813885 Review.
-
TRAIL-coated leukocytes that kill cancer cells in the circulation.Proc Natl Acad Sci U S A. 2014 Jan 21;111(3):930-5. doi: 10.1073/pnas.1316312111. Epub 2014 Jan 6. Proc Natl Acad Sci U S A. 2014. PMID: 24395803 Free PMC article.
-
The importance of nanoparticle shape in cancer drug delivery.Expert Opin Drug Deliv. 2015 Jan;12(1):129-42. doi: 10.1517/17425247.2014.950564. Epub 2014 Aug 20. Expert Opin Drug Deliv. 2015. PMID: 25138827 Review.
-
Biodegradable polymers for targeted delivery of anti-cancer drugs.Expert Opin Drug Deliv. 2016 Jun;13(6):891-909. doi: 10.1517/17425247.2016.1156671. Epub 2016 Mar 17. Expert Opin Drug Deliv. 2016. PMID: 26983898 Review.
-
Nanoparticle technologies for cancer therapy.Handb Exp Pharmacol. 2010;(197):55-86. doi: 10.1007/978-3-642-00477-3_2. Handb Exp Pharmacol. 2010. PMID: 20217526 Review.
Cited by
-
Engineering white blood cell membrane-camouflaged nanocarriers for inflammation-related therapeutics.Bioact Mater. 2022 Nov 9;23:80-100. doi: 10.1016/j.bioactmat.2022.10.026. eCollection 2023 May. Bioact Mater. 2022. PMID: 36406250 Free PMC article. Review.
-
Comprehensive comparison of theranostic nanoparticles in breast cancer.Am J Clin Exp Immunol. 2022 Feb 15;11(1):1-27. eCollection 2022. Am J Clin Exp Immunol. 2022. PMID: 35350450 Free PMC article. Review.
-
Human cytotoxic T-lymphocyte membrane-camouflaged nanoparticles combined with low-dose irradiation: a new approach to enhance drug targeting in gastric cancer.Int J Nanomedicine. 2017 Mar 17;12:2129-2142. doi: 10.2147/IJN.S126016. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28360520 Free PMC article.
-
Cell-mediated and cell membrane-coated nanoparticles for drug delivery and cancer therapy.Cancer Drug Resist. 2020;3(4):879-911. doi: 10.20517/cdr.2020.55. Epub 2020 Nov 3. Cancer Drug Resist. 2020. PMID: 33796822 Free PMC article.
-
Nanoparticles for Immune Cytokine TRAIL-Based Cancer Therapy.ACS Nano. 2018 Feb 27;12(2):912-931. doi: 10.1021/acsnano.7b05876. Epub 2018 Feb 6. ACS Nano. 2018. PMID: 29378114 Free PMC article. Review.
References
-
- Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–64. - PubMed
-
- Schroeder A, Heller DA, Winslow MM, et al. Treating metastatic cancer with nanotechnology. Nat Rev Cancer. 2011;12(1):39–50. This article broadly reviews current research, opportunities, and challenges in combining aspects of engineering, cancer biology, and medicine to develop nanotechnology platforms to treat metastatic cancer. - PubMed
-
- Safra T, Muggia F, Jeffers S, et al. Pegylated liposomal doxorubicin (doxil): reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m2. Ann Oncol. 2000;11:1029–33. - PubMed
-
- Christofori G. New signals from the invasive front. Nature. 2006;441(7092):444–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
