The α7 nicotinic receptor agonist ABT-107 protects against nigrostriatal damage in rats with unilateral 6-hydroxydopamine lesions
- PMID: 25261754
- PMCID: PMC4262739
- DOI: 10.1016/j.expneurol.2014.09.015
The α7 nicotinic receptor agonist ABT-107 protects against nigrostriatal damage in rats with unilateral 6-hydroxydopamine lesions
Abstract
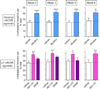
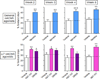
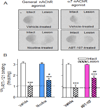
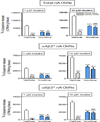
The finding that smoking is inversely correlated with Parkinson's disease and that nicotine attenuates nigrostriatal damage in Parkinsonian animals supports the idea that nicotine may be neuroprotective. Nicotine is thought to exert this effect by acting at nicotinic receptors (nAChRs), including the α7 subtype. The objective of this study was twofold: first, to test the protective potential of ABT-107, an agonist with high selectivity for α7 nAChRs; and second, to investigate its cellular mechanism of action. Rats were implanted with minipumps containing ABT-107 (0.25mg/kg/d). In addition, we tested the effect of nicotine (1mg/kg/d) as a positive control, and also DMXB (2mg/kg/d) which acts primarily with α7 but also α4β2* nAChRs. Two weeks after minipump placement, the rats were lesioned by unilateral administration of 6-hydroxydopamine (6-OHDA) into the medial forebrain bundle. Lesioning alone decreased contralateral forelimb use and adjusted stepping, two measures of Parkinsonism. ABT-107 and nicotine treatment significantly improved these behaviors at all weeks tested, with variable improvement with DMXB. We next investigated the cellular mechanism involved. The striatal dopamine transporter (DAT), a marker of dopaminergic integrity, was reduced ~70% with lesioning. ABT-107 or nicotine treatment significantly increased DAT levels in lesioned striatum; these drugs did not alter DAT levels in intact striatum. ABT-107 and nicotine also significantly improved basal dopamine release from lesioned striatum, as well as nicotine-stimulated dopamine release mediated via α4β2* and α6β2* nAChRs. These data suggest that α7 nAChR agonists may improve motor behaviors associated with nigrostriatal damage by enhancing striatal dopaminergic function.
Keywords: ABT-107; DMXB; Neuroprotection; Nicotine; Nicotinic receptors; Parkinson's disease.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
ABT-107 is an AbbVie compound; MWD is employed by and owns shares of AbbVie. There are no conflicts of interest for the other authors.
Figures


Similar articles
-
The nicotine-mediated decline in l-dopa-induced dyskinesias is associated with a decrease in striatal dopamine release.J Neurochem. 2013 Apr;125(2):291-302. doi: 10.1111/jnc.12179. Epub 2013 Mar 3. J Neurochem. 2013. PMID: 23373725 Free PMC article.
-
α7 nicotinic receptor agonists reduce levodopa-induced dyskinesias with severe nigrostriatal damage.Mov Disord. 2015 Dec;30(14):1901-1911. doi: 10.1002/mds.26453. Epub 2015 Nov 17. Mov Disord. 2015. PMID: 26573698 Free PMC article.
-
The α7 nicotinic receptor agonist ABT-107 decreases L-Dopa-induced dyskinesias in parkinsonian monkeys.J Pharmacol Exp Ther. 2014 Oct;351(1):25-32. doi: 10.1124/jpet.114.216283. Epub 2014 Jul 17. J Pharmacol Exp Ther. 2014. PMID: 25034405 Free PMC article.
-
α7 nicotinic acetylcholine receptor mediated neuroprotection in Parkinson's disease.Curr Drug Targets. 2012 May;13(5):623-30. doi: 10.2174/138945012800399026. Curr Drug Targets. 2012. PMID: 22300030 Review.
-
Multiple roles for nicotine in Parkinson's disease.Biochem Pharmacol. 2009 Oct 1;78(7):677-85. doi: 10.1016/j.bcp.2009.05.003. Epub 2009 May 9. Biochem Pharmacol. 2009. PMID: 19433069 Free PMC article. Review.
Cited by
-
Auricular Vagus Nerve Stimulation Exerts Antiinflammatory Effects and Immune Regulatory Function in a 6-OHDA Model of Parkinson's Disease.Neurochem Res. 2018 Nov;43(11):2155-2164. doi: 10.1007/s11064-018-2639-z. Epub 2018 Oct 11. Neurochem Res. 2018. PMID: 30311182
-
Prior nicotine self-administration attenuates subsequent dopaminergic deficits of methamphetamine in rats: role of nicotinic acetylcholine receptors.Behav Pharmacol. 2016 Aug;27(5):422-30. doi: 10.1097/FBP.0000000000000215. Behav Pharmacol. 2016. PMID: 26871405 Free PMC article.
-
Nicotinic modulation of glutamate receptor function at nerve terminal level: a fine-tuning of synaptic signals.Front Pharmacol. 2015 Apr 29;6:89. doi: 10.3389/fphar.2015.00089. eCollection 2015. Front Pharmacol. 2015. PMID: 25972809 Free PMC article. Review.
-
Nicotinic ligands as multifunctional agents for the treatment of neuropsychiatric disorders.Biochem Pharmacol. 2015 Oct 15;97(4):388-398. doi: 10.1016/j.bcp.2015.07.027. Epub 2015 Jul 29. Biochem Pharmacol. 2015. PMID: 26231940 Free PMC article. Review.
-
The neuroprotective effect of nicotine in Parkinson's disease models is associated with inhibiting PARP-1 and caspase-3 cleavage.PeerJ. 2017 Oct 19;5:e3933. doi: 10.7717/peerj.3933. eCollection 2017. PeerJ. 2017. PMID: 29062606 Free PMC article.
References
-
- Bitner RS, et al. In vivo pharmacological characterization of a novel selective alpha7 neuronal nicotinic acetylcholine receptor agonist ABT-107: preclinical considerations in Alzheimer's disease. J Pharmacol Exp Ther. 2010;334:875–886. - PubMed
-
- Bordia T, et al. Continuous and intermittent nicotine treatment reduces L-3,4-dihydroxyphenylalanine (L-DOPA)-induced dyskinesias in a rat model of Parkinson's disease. J Pharmacol Exp Ther. 2008;327:239–247. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

