Preconditioning of bone marrow mesenchymal stem cells by prolyl hydroxylase inhibition enhances cell survival and angiogenesis in vitro and after transplantation into the ischemic heart of rats
- PMID: 25257482
- PMCID: PMC4535299
- DOI: 10.1186/scrt499
Preconditioning of bone marrow mesenchymal stem cells by prolyl hydroxylase inhibition enhances cell survival and angiogenesis in vitro and after transplantation into the ischemic heart of rats
Abstract
Introduction: Poor cell survival and limited functional benefits have restricted the efficacy of bone marrow mesenchymal stem cells (BMSCs) in the treatment of myocardial infarction. We showed recently that hypoxia preconditioning of BMSCs and neural progenitor cells before transplantation can enhance the survival and therapeutic properties of these cells in the ischemic brain and heart. The present investigation explores a novel strategy of preconditioning BMSCs using the Hypoxia-inducible factor 1α (HIF-α) prolyl hydroxylase inhibitor dimethyloxalylglycine (DMOG) to enhance their survival and therapeutic efficacy after transplantation into infarcted myocardium.
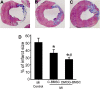
Methods: BMSCs from green fluorescent protein transgenic rats were cultured with or without 1 mM DMOG for 24 hours in complete culture medium before transplantation. Survival and angiogenic factors were evaluated in vitro by trypan blue staining, Western blotting, and tube formation test. In an ischemic heart model of rats, BMSCs with and without DMOG preconditioning were intramyocardially transplanted into the peri-infarct region 30 minutes after permanent myocardial ischemia. Cell death was measured 24 hours after engraftment. Heart function, angiogenesis and infarct size were measured 4 weeks later.
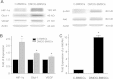
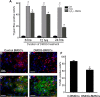
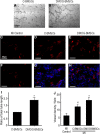
Results: In DMOG preconditioned BMSCs (DMOG-BMSCs), the expression of survival and angiogenic factors including HIF-1α, vascular endothelial growth factor, glucose transporter 1 and phospho-Akt were significantly increased. In comparison with control cells, DMOG-BMSCs showed higher viability and enhanced angiogenesis in both in vitro and in vivo assays. Transplantation of DMOG-BMSCs reduced heart infarct size and promoted functional benefits of the cell therapy.
Conclusions: We suggest that DMOG preconditioning enhances the survival capability of BMSCs and paracrine effects with increased differentiation potential. Prolyl hydroxylase inhibition is an effective and feasible strategy to enhance therapeutic efficacy and efficiency of BMSC transplantation therapy after heart ischemia.
Figures
Similar articles
-
Cellular repressor of E1A-stimulated gene overexpression in bone mesenchymal stem cells protects against rat myocardial infarction.Int J Cardiol. 2015 Mar 15;183:232-41. doi: 10.1016/j.ijcard.2015.01.059. Epub 2015 Jan 27. Int J Cardiol. 2015. PMID: 25679992
-
Myocardium-targeted transplantation of PHD2 shRNA-modified bone mesenchymal stem cells through ultrasound-targeted microbubble destruction protects the heart from acute myocardial infarction.Theranostics. 2020 Apr 6;10(11):4967-4982. doi: 10.7150/thno.43233. eCollection 2020. Theranostics. 2020. PMID: 32308762 Free PMC article.
-
Dimethyloxalylglycine preconditioning enhances protective effects of bone marrow-derived mesenchymal stem cells in Aβ- induced Alzheimer disease.Physiol Behav. 2019 Feb 1;199:265-272. doi: 10.1016/j.physbeh.2018.11.034. Epub 2018 Nov 27. Physiol Behav. 2019. PMID: 30500334
-
Effect of Dimethyloxalylglycine on Stem Cells Osteogenic Differentiation and Bone Tissue Regeneration-A Systematic Review.Int J Mol Sci. 2024 Mar 30;25(7):3879. doi: 10.3390/ijms25073879. Int J Mol Sci. 2024. PMID: 38612687 Free PMC article. Review.
-
Bone marrow stromal cell transplantation for ischemic stroke -- its multi-functional feature.Acta Neurobiol Exp (Wars). 2013;73(1):57-65. doi: 10.55782/ane-2013-1921. Acta Neurobiol Exp (Wars). 2013. PMID: 23595283 Review.
Cited by
-
Hypoxia-Inducible Factor-1 in Physiological and Pathophysiological Angiogenesis: Applications and Therapies.Biomed Res Int. 2015;2015:549412. doi: 10.1155/2015/549412. Epub 2015 Jun 4. Biomed Res Int. 2015. PMID: 26146622 Free PMC article. Review.
-
Dimethyloxaloylglycine Promotes the Angiogenic Activity of Mesenchymal Stem Cells Derived from iPSCs via Activation of the PI3K/Akt Pathway for Bone Regeneration.Int J Biol Sci. 2016 Apr 8;12(6):639-52. doi: 10.7150/ijbs.14025. eCollection 2016. Int J Biol Sci. 2016. PMID: 27194942 Free PMC article.
-
Preconditioning with far-infrared irradiation enhances proliferation, cell survival, and migration of rat bone marrow-derived stem cells via CXCR4-ERK pathways.Sci Rep. 2017 Oct 20;7(1):13718. doi: 10.1038/s41598-017-14219-w. Sci Rep. 2017. PMID: 29057951 Free PMC article.
-
Stem Cell Metabolism: Powering Cell-Based Therapeutics.Cells. 2020 Nov 16;9(11):2490. doi: 10.3390/cells9112490. Cells. 2020. PMID: 33207756 Free PMC article. Review.
-
Mapping current research and identifying hotspots on mesenchymal stem cells in cardiovascular disease.Stem Cell Res Ther. 2020 Nov 25;11(1):498. doi: 10.1186/s13287-020-02009-7. Stem Cell Res Ther. 2020. PMID: 33239082 Free PMC article.
References
-
- Braunwald E, Bristow MR. Congestive heart failure: fifty years of progress. Circulation. 2000;102:IV14–IV23. - PubMed
-
- Bartunek J, Croissant JD, Wijns W, Gofflot S, de Lavareille A, Vanderheyden M, Kaluzhny Y, Mazouz N, Willemsen P, Penicka M, Mathieu M, Homsy C, De Bruyne B, McEntee K, Lee IW, Heyndrickx GR. Pretreatment of adult bone marrow mesenchymal stem cells with cardiomyogenic growth factors and repair of the chronically infarcted myocardium. Am J Physiol Heart Circ Physiol. 2007;292:H1095–H1104. doi: 10.1152/ajpheart.01009.2005. - DOI - PubMed
-
- Behfar A, Yamada S, Crespo-Diaz R, Nesbitt JJ, Rowe LA, Perez-Terzic C, Gaussin V, Homsy C, Bartunek J, Terzic A. Guided cardiopoiesis enhances therapeutic benefit of bone marrow human mesenchymal stem cells in chronic myocardial infarction. J Am Coll Cardiol. 2010;56:721–734. doi: 10.1016/j.jacc.2010.03.066. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

