Maternal LPS exposure during pregnancy impairs testicular development, steroidogenesis and spermatogenesis in male offspring
- PMID: 25255222
- PMCID: PMC4177809
- DOI: 10.1371/journal.pone.0106786
Maternal LPS exposure during pregnancy impairs testicular development, steroidogenesis and spermatogenesis in male offspring
Abstract
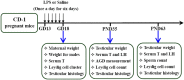
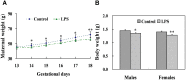
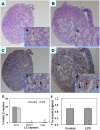
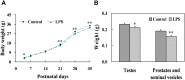
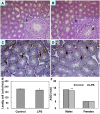
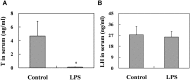
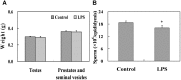
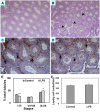
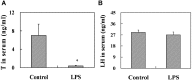
Lipopolysaccharide (LPS) is associated with adverse developmental outcomes including embryonic resorption, fetal death, congenital teratogenesis and fetal growth retardation. Here, we explored the effects of maternal LPS exposure during pregnancy on testicular development, steroidogenesis and spermatogenesis in male offspring. The pregnant mice were intraperitoneally injected with LPS (50 µg/kg) daily from gestational day (GD) 13 to GD 17. At fetal period, a significant decrease in body weight and abnormal Leydig cell aggregations were observed in males whose mothers were exposed to LPS during pregnancy. At postnatal day (PND) 26, anogenital distance (AGD), a sensitive index of altered androgen action, was markedly reduced in male pups whose mothers were exposed to LPS daily from GD13 to GD 17. At PND35, the weight of testes, prostates and seminal vesicles, and serum testosterone (T) level were significantly decreased in LPS-treated male pups. At adulthood, the number of sperm was significantly decreased in male offspring whose mothers were exposed to LPS on GD 13-17. Maternal LPS exposure during gestation obviously diminished the percent of seminiferous tubules in stages I-VI, increased the percent of seminiferous tubules in stages IX-XII, and caused massive sloughing of germ cells in seminiferous tubules in mouse testes. Moreover, maternal LPS exposure significantly reduced serum T level in male mice whose mothers were exposed to LPS challenge during pregnancy. Taken together, these results suggest that maternal LPS exposure during pregnancy disrupts T production. The decreased T synthesis might be associated with LPS-induced impairments for spermatogenesis in male offspring.
Conflict of interest statement
Figures
Similar articles
-
Lactational fenvalerate exposure permanently impairs testicular development and spermatogenesis in mice.Toxicol Lett. 2009 Dec 1;191(1):47-56. doi: 10.1016/j.toxlet.2009.08.007. Epub 2009 Aug 14. Toxicol Lett. 2009. PMID: 19683566
-
Maternal cypermethrin exposure during lactation impairs testicular development and spermatogenesis in male mouse offspring.Environ Toxicol. 2011 Aug;26(4):382-94. doi: 10.1002/tox.20566. Epub 2010 Feb 3. Environ Toxicol. 2011. PMID: 20131380
-
Maternal fenvalerate exposure during pregnancy persistently impairs testicular development and spermatogenesis in male offspring.Food Chem Toxicol. 2010 May;48(5):1160-9. doi: 10.1016/j.fct.2010.02.003. Epub 2010 Feb 6. Food Chem Toxicol. 2010. PMID: 20138952
-
Analgesic use in pregnancy and male reproductive development.Curr Opin Endocrinol Diabetes Obes. 2017 Jun;24(3):225-232. doi: 10.1097/MED.0000000000000338. Curr Opin Endocrinol Diabetes Obes. 2017. PMID: 28277341 Free PMC article. Review.
-
Possible fetal determinants of male infertility.Nat Rev Endocrinol. 2014 Sep;10(9):553-62. doi: 10.1038/nrendo.2014.97. Epub 2014 Jun 17. Nat Rev Endocrinol. 2014. PMID: 24935122 Review.
Cited by
-
Dysbacteriosis-Derived Lipopolysaccharide Causes Embryonic Osteopenia through Retinoic-Acid-Regulated DLX5 Expression.Int J Mol Sci. 2020 Apr 4;21(7):2518. doi: 10.3390/ijms21072518. Int J Mol Sci. 2020. PMID: 32260461 Free PMC article.
-
Gestational diabetes exacerbates intrauterine microbial exposure induced intestinal microbiota change in offspring contributing to increased immune response.Nutr Diabetes. 2024 Oct 19;14(1):87. doi: 10.1038/s41387-024-00346-7. Nutr Diabetes. 2024. PMID: 39424815 Free PMC article.
-
Zinc-nanoparticles alleviate the ovarian damage induced by bacterial lipopolysaccharide (LPS) in pregnant rats and their fetuses.Histochem Cell Biol. 2023 Nov;160(5):453-475. doi: 10.1007/s00418-023-02222-4. Epub 2023 Jul 26. Histochem Cell Biol. 2023. PMID: 37495867 Free PMC article.
-
Adrenomedullin protects Leydig cells against lipopolysaccharide-induced oxidative stress and inflammatory reaction via MAPK/NF-κB signalling pathways.Sci Rep. 2017 Nov 28;7(1):16479. doi: 10.1038/s41598-017-16008-x. Sci Rep. 2017. PMID: 29184072 Free PMC article.
-
Effects of Dietary Fat Sources during Late Gestation on Colostrum Quality and Mammary Gland Inflammation in Lipopolysaccharide-Challenged Sows.Animals (Basel). 2020 Feb 18;10(2):319. doi: 10.3390/ani10020319. Animals (Basel). 2020. PMID: 32085517 Free PMC article.
References
-
- Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG (1995) Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology 108: 218–224. - PubMed
-
- Platz-Christensen JJ, Mattsby-Baltzer I, Thomsen P, Wiqvist N (1993) Endotoxin and interleukin-1 alpha in the cervical mucus and vaginal fluid of pregnant women with bacterial vaginosis. Am J Obstet Gynecol 169: 1161–1166. - PubMed
-
- Romero R, Roslansky P, Oyarzun E, Wan M, Emamian M, et al. (1988) Labor and infection. II. Bacterial endotoxin in amniotic fluid and its relationship to the onset of preterm labor. Am J Obstet Gynecol 158: 1044–1049. - PubMed
-
- Hazan Y, Mazor M, Horowitz S, Leiberman JR, Glezerman M (1995) The diagnostic value of amniotic fluid Gram stain examination and limulus amebocyte lysate assay in patients with preterm birth. Acta Obstet Gynecol Scand 74: 275–280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

