γ-Interferon-inducible lysosomal thiol reductase (GILT) maintains phagosomal proteolysis in alternatively activated macrophages
- PMID: 25253686
- PMCID: PMC4231668
- DOI: 10.1074/jbc.M114.584391
γ-Interferon-inducible lysosomal thiol reductase (GILT) maintains phagosomal proteolysis in alternatively activated macrophages
Abstract
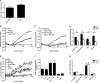
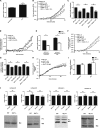
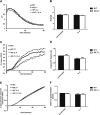
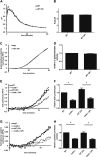
Although it is known that lysosomal cysteine cathepsins require a reducing environment for optimal activity, it is not firmly established how these enzymes are maintained in their reduced-active state in the acidic and occasionally oxidative environment within phagosomes and lysosomes. γ-Interferon-inducible lysosomal thiol reductase (GILT) has been the only enzyme described in the endosomes, lysosomes, and phagosomes with the potential to catalyze the reduction of cysteine cathepsins. Our goal in the current study was to assess the effect of GILT on major phagosomal functions with an emphasis on proteolytic efficiency in murine bone marrow-derived macrophages. Assessment of phagosomal disulfide reduction upon internalization of IgG-opsonized experimental particles confirmed a major role for GILT in phagosomal disulfide reduction in both resting and interferon-γ-activated macrophages. Furthermore we observed a decrease in early phagosomal proteolytic efficiency in GILT-deficient macrophages, specifically in the absence of an NADPH oxidase-mediated respiratory burst. This deficiency was more prominent in IL-4-activated macrophages that inherently possess lower levels of NADPH oxidase activity. Finally, we provide evidence that GILT is required for optimal activity of the lysosomal cysteine protease, cathepsin S. In summary, our results suggest a role for GILT in maintaining cysteine cathepsin proteolytic efficiency in phagosomes, particularly in the absence of high NADPH oxidase activity, which is characteristic of alternatively activated macrophages.
Keywords: Cysteine Protease; Lysosome; Macrophage; Oxidation-Reduction (Redox); Phagosome; Proteolysis; Reductase.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

Similar articles
-
Ligation of FcγR Alters Phagosomal Processing of Protein via Augmentation of NADPH Oxidase Activity.Traffic. 2016 Jul;17(7):786-802. doi: 10.1111/tra.12396. Epub 2016 May 13. Traffic. 2016. PMID: 27020146
-
Endogenous and exogenous pathways maintain the reductive capacity of the phagosome.J Leukoc Biol. 2016 Jul;100(1):17-26. doi: 10.1189/jlb.2HI0315-083R. Epub 2015 Dec 28. J Leukoc Biol. 2016. PMID: 26710800
-
NADPH oxidase activity controls phagosomal proteolysis in macrophages through modulation of the lumenal redox environment of phagosomes.Proc Natl Acad Sci U S A. 2010 Jun 8;107(23):10496-501. doi: 10.1073/pnas.0914867107. Epub 2010 May 24. Proc Natl Acad Sci U S A. 2010. PMID: 20498052 Free PMC article.
-
The phagosome and redox control of antigen processing.Free Radic Biol Med. 2018 Sep;125:53-61. doi: 10.1016/j.freeradbiomed.2018.03.040. Epub 2018 Mar 22. Free Radic Biol Med. 2018. PMID: 29578071 Review.
-
Disulfide reduction in the endocytic pathway: immunological functions of gamma-interferon-inducible lysosomal thiol reductase.Antioxid Redox Signal. 2011 Aug 1;15(3):657-68. doi: 10.1089/ars.2010.3684. Epub 2011 Apr 20. Antioxid Redox Signal. 2011. PMID: 21506690 Free PMC article. Review.
Cited by
-
A physiologic overview of the organ-specific transcriptome of the cattle tick Rhipicephalus microplus.Sci Rep. 2020 Oct 26;10(1):18296. doi: 10.1038/s41598-020-75341-w. Sci Rep. 2020. PMID: 33106528 Free PMC article.
-
Biguanide is a modifiable pharmacophore for recruitment of endogenous Zn2+ to inhibit cysteinyl cathepsins: review and implications.Biometals. 2019 Aug;32(4):575-593. doi: 10.1007/s10534-019-00197-1. Epub 2019 May 1. Biometals. 2019. PMID: 31044334 Free PMC article. Review.
-
Approaches to Measuring Reductive and Oxidative Events in Phagosomes.Methods Mol Biol. 2023;2692:139-152. doi: 10.1007/978-1-0716-3338-0_10. Methods Mol Biol. 2023. PMID: 37365466
-
Growth hormone-mediated reprogramming of macrophage transcriptome and effector functions.Sci Rep. 2019 Dec 18;9(1):19348. doi: 10.1038/s41598-019-56017-6. Sci Rep. 2019. PMID: 31852980 Free PMC article.
-
Measuring Phagosome pH by Ratiometric Fluorescence Microscopy.J Vis Exp. 2015 Dec 7;(106):e53402. doi: 10.3791/53402. J Vis Exp. 2015. PMID: 26710109 Free PMC article.
References
-
- Nunes P., Demaurex N., Dinauer M. C. (2013) Regulation of the NADPH oxidase and associated ion fluxes during phagocytosis. Traffic 14, 1118–1131 - PubMed
-
- Flannagan R. S., Cosío G., Grinstein S. (2009) Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat. Rev. Microbiol. 7, 355–366 - PubMed
-
- Claus V., Jahraus A., Tjelle T., Berg T., Kirschke H., Faulstich H., Griffiths G. (1998) Lysosomal enzyme trafficking between phagosomes, endosomes, and lysosomes in J774 macrophages. Enrichment of cathepsin H in early endosomes. J. Biol. Chem. 273, 9842–9851 - PubMed
-
- Kotsias F., Hoffmann E., Amigorena S., Savina A. (2013) Reactive oxygen species production in the phagosome: impact on antigen presentation in dendritic cells. Antioxid. Redox Signal. 18, 714–729 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

