Functional mitochondrial analysis in acute brain sections from adult rats reveals mitochondrial dysfunction in a rat model of migraine
- PMID: 25252946
- PMCID: PMC4254950
- DOI: 10.1152/ajpcell.00332.2013
Functional mitochondrial analysis in acute brain sections from adult rats reveals mitochondrial dysfunction in a rat model of migraine
Abstract
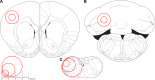
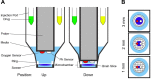
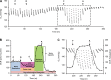
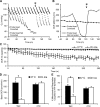
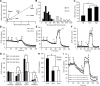
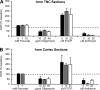
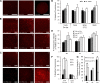
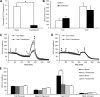
Mitochondrial dysfunction has been implicated in many neurological disorders that only develop or are much more severe in adults, yet no methodology exists that allows for medium-throughput functional mitochondrial analysis of brain sections from adult animals. We developed a technique for quantifying mitochondrial respiration in acutely isolated adult rat brain sections with the Seahorse XF Analyzer. Evaluating a range of conditions made quantifying mitochondrial function from acutely derived adult brain sections from the cortex, cerebellum, and trigeminal nucleus caudalis possible. Optimization of this technique demonstrated that the ideal section size was 1 mm wide. We found that sectioning brains at physiological temperatures was necessary for consistent metabolic analysis of trigeminal nucleus caudalis sections. Oxygen consumption in these sections was highly coupled to ATP synthesis, had robust spare respiratory capacities, and had limited nonmitochondrial respiration, all indicative of healthy tissue. We demonstrate the effectiveness of this technique by identifying a decreased spare respiratory capacity in the trigeminal nucleus caudalis of a rat model of chronic migraine, a neurological disorder that has been associated with mitochondrial dysfunction. This technique allows for 24 acutely isolated sections from multiple brain regions of a single adult rat to be analyzed simultaneously with four sequential drug treatments, greatly advancing the ability to study mitochondrial physiology in adult neurological disorders.
Keywords: adult brain sections; brain energy metabolism; mitochondria; neurological disorders; neuron-glial interactions.
Copyright © 2014 the American Physiological Society.
Figures
Similar articles
-
Distinct differences in rates of oxygen consumption and ATP synthesis of regionally isolated non-synaptic mouse brain mitochondria.J Neurosci Res. 2019 Aug;97(8):961-974. doi: 10.1002/jnr.24371. Epub 2019 Jan 24. J Neurosci Res. 2019. PMID: 30675904
-
PTEN inhibition preserves trigeminal nucleus caudalis neuron activation through tyrosine phosphorylation of the NR2B subunit at Tyr1472 of the NMDA receptor in a rat model of recurrent migraine.Neurol Res. 2016 Apr;38(4):320-6. doi: 10.1080/01616412.2016.1145915. Epub 2016 Apr 27. Neurol Res. 2016. PMID: 27121999
-
Impaired Mitochondrial Bioenergetics Function in Pediatric Chronic Overlapping Pain Conditions with Functional Gastrointestinal Disorders.Pain Res Manag. 2021 Aug 13;2021:6627864. doi: 10.1155/2021/6627864. eCollection 2021. Pain Res Manag. 2021. PMID: 34426756 Free PMC article.
-
[Migraine and mitochondrial dysfunction].Rev Neurol (Paris). 1996 Apr;152(4):234-8. Rev Neurol (Paris). 1996. PMID: 8763651 Review. French.
-
CoEnzyme Q10 and riboflavin: the mitochondrial connection.Headache. 2012 Oct;52 Suppl 2:81-7. doi: 10.1111/j.1526-4610.2012.02233.x. Headache. 2012. PMID: 23030537 Review.
Cited by
-
A method for assessing tissue respiration in anatomically defined brain regions.Sci Rep. 2020 Aug 6;10(1):13179. doi: 10.1038/s41598-020-69867-2. Sci Rep. 2020. PMID: 32764697 Free PMC article.
-
Voluntary exercise delays progressive deterioration of markers of metabolism and behavior in a mouse model of Parkinson's disease.Brain Res. 2019 Oct 1;1720:146301. doi: 10.1016/j.brainres.2019.146301. Epub 2019 Jun 18. Brain Res. 2019. PMID: 31226324 Free PMC article. Review.
-
Protective Roles of N-acetyl Cysteine and/or Taurine against Sumatriptan-Induced Hepatotoxicity.Adv Pharm Bull. 2016 Dec;6(4):627-637. doi: 10.15171/apb.2016.077. Epub 2016 Dec 22. Adv Pharm Bull. 2016. PMID: 28101470 Free PMC article.
-
The Role of Metabolism in Migraine Pathophysiology and Susceptibility.Life (Basel). 2021 May 1;11(5):415. doi: 10.3390/life11050415. Life (Basel). 2021. PMID: 34062792 Free PMC article. Review.
-
Mild traumatic brain injury increases vulnerability to cerebral ischemia in mice.Exp Neurol. 2021 Aug;342:113765. doi: 10.1016/j.expneurol.2021.113765. Epub 2021 May 13. Exp Neurol. 2021. PMID: 33992581 Free PMC article.
References
-
- Allaman I, Bélanger M, Magistretti PJ. Astrocyte-neuron metabolic relationships: For better and for worse. Trends Neurosci 34: 76–87, 2011. - PubMed
-
- Blättler SM, Verdeguer F, Liesa M, Cunningham JT, Vogel RO, Chim H, Liu H, Romanino K, Shirihai OS, Vazquez F, Rüegg MA, Shi Y, Puigserver P. Defective mitochondrial morphology and bioenergetic function in mice lacking the transcription factor Yin Yang 1 in skeletal muscle. Mol Cell Biol 32: 3333–3346, 2012. - PMC - PubMed
-
- Bogousslavsky J, Meienberg O. EYe-movement disorders in brain-stem and cerebellar stroke. Arch Neurol 44: 141–148, 1987. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

