Functional implications of mitofusin 2-mediated mitochondrial-SR tethering
- PMID: 25252175
- PMCID: PMC4268321
- DOI: 10.1016/j.yjmcc.2014.09.015
Functional implications of mitofusin 2-mediated mitochondrial-SR tethering
Abstract
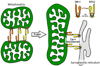
Cardiomyocyte mitochondria have an intimate physical and functional relationship with sarcoplasmic reticulum (SR). Under normal conditions mitochondrial ATP is essential to power SR calcium cycling that drives phasic contraction/relaxation, and changes in SR calcium release are sensed by mitochondria and used to modulate oxidative phosphorylation according to metabolic need. When perturbed, mitochondrial-SR calcium crosstalk can evoke programmed cell death. Physical proximity and functional interplay between mitochondria and SR are maintained in part through tethering of these two organelles by the membrane protein mitofusin 2 (Mfn2). Here we review and discuss findings from our two laboratories that derive from genetic manipulation of Mfn2 and closely related Mfn1 in mouse hearts and other experimental systems. By comparing the findings of our two independent research efforts we arrive at several conclusions that appear to be strongly supported, and describe a few areas of incomplete understanding that will require further study. In so doing we hope to clarify some misconceptions regarding the many varied roles of Mfn2 as both physical trans-organelle tether and mitochondrial fusion protein. This article is part of a Special Issue entitled "Mitochondria: From Basic Mitochondrial Biology to Cardiovascular Disease."
Keywords: Calcium cross-talk; Mitochondria; Mitochondrial fusion; Mitochondrial permeability transition pore; Organelle tethering; Sarcoplasmic reticulum.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Hearts deficient in both Mfn1 and Mfn2 are protected against acute myocardial infarction.Cell Death Dis. 2016 May 26;7(5):e2238. doi: 10.1038/cddis.2016.139. Cell Death Dis. 2016. PMID: 27228353 Free PMC article.
-
Mitofusin 2-containing mitochondrial-reticular microdomains direct rapid cardiomyocyte bioenergetic responses via interorganelle Ca(2+) crosstalk.Circ Res. 2012 Sep 14;111(7):863-75. doi: 10.1161/CIRCRESAHA.112.266585. Epub 2012 Jul 9. Circ Res. 2012. PMID: 22777004 Free PMC article.
-
Role of Mitofusin-2 in mitochondrial localization and calcium uptake in skeletal muscle.Cell Calcium. 2015 Jan;57(1):14-24. doi: 10.1016/j.ceca.2014.11.002. Epub 2014 Nov 15. Cell Calcium. 2015. PMID: 25477138 Free PMC article.
-
Mitochondrial dynamism and cardiac fate--a personal perspective.Circ J. 2013;77(6):1370-9. doi: 10.1253/circj.cj-13-0453. Epub 2013 Apr 25. Circ J. 2013. PMID: 23615052 Review.
-
SR and mitochondria: calcium cross-talk between kissing cousins.J Mol Cell Cardiol. 2013 Feb;55:42-9. doi: 10.1016/j.yjmcc.2012.07.015. Epub 2012 Aug 2. J Mol Cell Cardiol. 2013. PMID: 22902320 Review.
Cited by
-
New therapeutics to modulate mitochondrial dynamics and mitophagy in cardiac diseases.J Mol Med (Berl). 2015 Mar;93(3):279-87. doi: 10.1007/s00109-015-1256-4. Epub 2015 Feb 5. J Mol Med (Berl). 2015. PMID: 25652199 Free PMC article. Review.
-
Modifications of Mitochondrial Network Morphology Affect the MAVS-Dependent Immune Response in L929 Murine Fibroblasts during Ectromelia Virus Infection.Pathogens. 2024 Aug 23;13(9):717. doi: 10.3390/pathogens13090717. Pathogens. 2024. PMID: 39338909 Free PMC article.
-
Mitochondrial responses to intracellular Ca2+ release following infrared stimulation.J Neurophysiol. 2023 Mar 1;129(3):700-716. doi: 10.1152/jn.00293.2022. Epub 2023 Feb 8. J Neurophysiol. 2023. PMID: 36752512 Free PMC article.
-
Pharmacological Modulation of Mitochondrial Ca2+ Content Regulates Sarcoplasmic Reticulum Ca2+ Release via Oxidation of the Ryanodine Receptor by Mitochondria-Derived Reactive Oxygen Species.Front Physiol. 2018 Dec 21;9:1831. doi: 10.3389/fphys.2018.01831. eCollection 2018. Front Physiol. 2018. PMID: 30622478 Free PMC article.
-
Sarcoplasmic reticulum-mitochondria communication; implications for cardiac arrhythmia.J Mol Cell Cardiol. 2021 Jul;156:105-113. doi: 10.1016/j.yjmcc.2021.04.002. Epub 2021 Apr 17. J Mol Cell Cardiol. 2021. PMID: 33857485 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

