Phospholipase D signaling pathways and phosphatidic acid as therapeutic targets in cancer
- PMID: 25244928
- PMCID: PMC4180337
- DOI: 10.1124/pr.114.009217
Phospholipase D signaling pathways and phosphatidic acid as therapeutic targets in cancer
Abstract
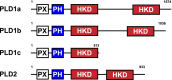
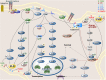
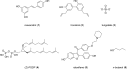
Phospholipase D is a ubiquitous class of enzymes that generates phosphatidic acid as an intracellular signaling species. The phospholipase D superfamily plays a central role in a variety of functions in prokaryotes, viruses, yeast, fungi, plants, and eukaryotic species. In mammalian cells, the pathways modulating catalytic activity involve a variety of cellular signaling components, including G protein-coupled receptors, receptor tyrosine kinases, polyphosphatidylinositol lipids, Ras/Rho/ADP-ribosylation factor GTPases, and conventional isoforms of protein kinase C, among others. Recent findings have shown that phosphatidic acid generated by phospholipase D plays roles in numerous essential cellular functions, such as vesicular trafficking, exocytosis, autophagy, regulation of cellular metabolism, and tumorigenesis. Many of these cellular events are modulated by the actions of phosphatidic acid, and identification of two targets (mammalian target of rapamycin and Akt kinase) has especially highlighted a role for phospholipase D in the regulation of cellular metabolism. Phospholipase D is a regulator of intercellular signaling and metabolic pathways, particularly in cells that are under stress conditions. This review provides a comprehensive overview of the regulation of phospholipase D activity and its modulation of cellular signaling pathways and functions.
Copyright © 2014 by The American Society for Pharmacology and Experimental Therapeutics.
Figures
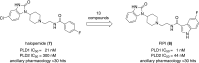
Similar articles
-
Structure and regulation of human phospholipase D.Adv Biol Regul. 2021 Jan;79:100783. doi: 10.1016/j.jbior.2020.100783. Epub 2021 Jan 3. Adv Biol Regul. 2021. PMID: 33495125 Free PMC article. Review.
-
Phospholipase D.Biochem Cell Biol. 2004 Feb;82(1):225-53. doi: 10.1139/o03-079. Biochem Cell Biol. 2004. PMID: 15052340 Review.
-
Phospholipase D: a lipid centric review.Cell Mol Life Sci. 2005 Oct;62(19-20):2305-16. doi: 10.1007/s00018-005-5195-z. Cell Mol Life Sci. 2005. PMID: 16143829 Free PMC article. Review.
-
Phospholipid signalling through phospholipase D and phosphatidic acid.IUBMB Life. 2006 Aug;58(8):457-61. doi: 10.1080/15216540600871142. IUBMB Life. 2006. PMID: 16916782 Review.
-
Phosphatidic acid signaling regulation of Ras superfamily of small guanosine triphosphatases.Biochim Biophys Acta. 2009 Sep;1791(9):850-5. doi: 10.1016/j.bbalip.2009.05.013. Epub 2009 Jun 21. Biochim Biophys Acta. 2009. PMID: 19540930 Free PMC article. Review.
Cited by
-
Structural insights into PA3488-mediated inactivation of Pseudomonas aeruginosa PldA.Nat Commun. 2022 Oct 10;13(1):5979. doi: 10.1038/s41467-022-33690-2. Nat Commun. 2022. PMID: 36216841 Free PMC article.
-
Ritanserin suppresses acute myeloid leukemia by inhibiting DGKα to downregulate phospholipase D and the Jak-Stat/MAPK pathway.Discov Oncol. 2023 Jul 1;14(1):118. doi: 10.1007/s12672-023-00737-9. Discov Oncol. 2023. PMID: 37392305 Free PMC article.
-
Nir1-LNS2 is a novel phosphatidic acid biosensor that reveals mechanisms of lipid production.bioRxiv [Preprint]. 2024 Feb 28:2024.02.28.582557. doi: 10.1101/2024.02.28.582557. bioRxiv. 2024. PMID: 38464273 Free PMC article. Preprint.
-
Structure and regulation of human phospholipase D.Adv Biol Regul. 2021 Jan;79:100783. doi: 10.1016/j.jbior.2020.100783. Epub 2021 Jan 3. Adv Biol Regul. 2021. PMID: 33495125 Free PMC article. Review.
-
Phosphatidic acid-mediated binding and mammalian cell internalization of the Vibrio cholerae cytotoxin MakA.PLoS Pathog. 2021 Mar 18;17(3):e1009414. doi: 10.1371/journal.ppat.1009414. eCollection 2021 Mar. PLoS Pathog. 2021. PMID: 33735319 Free PMC article.
References
-
- Adachi T, Nakashima S, Saji S, Nakamura T, Nozawa Y. (1996) Phospholipase D activation in hepatocyte growth factor-stimulated rat hepatocytes mediates the expressions of c-jun and c-protein: involvement of protein tyrosine kinase, protein kinase C, and Ca2+. Hepatology 24:1274–1281 - PubMed
-
- Aguirre Ghiso JA, Farías EF, Alonso DF, Arregui C, Bal de Kier Joffé E. (1997) A phospholipase D and protein kinase C inhibitor blocks the spreading of murine mammary adenocarcinoma cells altering f-actin and beta1-integrin point contact distribution. Int J Cancer 71:881–890 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
