Loss of monocyte chemoattractant protein-1 alters macrophage polarization and reduces NFκB activation in the foreign body response
- PMID: 25242651
- PMCID: PMC4278755
- DOI: 10.1016/j.actbio.2014.09.022
Loss of monocyte chemoattractant protein-1 alters macrophage polarization and reduces NFκB activation in the foreign body response
Abstract
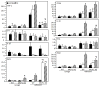
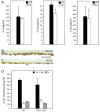
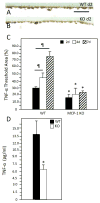
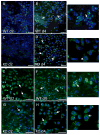
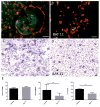
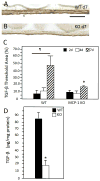
Implantation of biomaterials elicits a foreign body response characterized by fusion of macrophages to form foreign body giant cells and fibrotic encapsulation. Studies of the macrophage polarization involved in this response have suggested that alternative (M2) activation is associated with more favorable outcomes. Here we investigated this process in vivo by implanting mixed cellulose ester filters or polydimethylsiloxane disks in the peritoneal cavity of wild-type (WT) and monocyte chemoattractant protein-1 (MCP-1) knockout mice. We analyzed classical (M1) and alternative (M2) gene expression via quantitative polymerase chain reaction, immunohistochemistry and enzyme-linked immunosorbent assay in both non-adherent cells isolated by lavage and implant-adherent cells. Our results show that macrophages undergo unique activation that displays features of both M1 and M2 polarization including induction of tumor necrosis factor α (TNF), which induces the expression and nuclear translocation of p50 and RelA determined by immunofluorescence and Western blot. Both processes were compromised in fusion-deficient MCP-1 KO macrophages in vitro and in vivo. Furthermore, inclusion of BAY 11-7028, an inhibitor of NFκB activation, reduced nuclear translocation of RelA and fusion in WT macrophages. Our studies suggest that peritoneal implants elicit a unique macrophage polarization phenotype leading to induction of TNF and activation of the NFκB pathway.
Keywords: Foreign body giant cell; Foreign body response; Inflammation; Macrophage.
Published by Elsevier Ltd.
Figures

Similar articles
-
Hydrogen sulfide suppresses oxidized low-density lipoprotein (ox-LDL)-stimulated monocyte chemoattractant protein 1 generation from macrophages via the nuclear factor κB (NF-κB) pathway.J Biol Chem. 2014 Apr 4;289(14):9741-53. doi: 10.1074/jbc.M113.517995. Epub 2014 Feb 18. J Biol Chem. 2014. PMID: 24550391 Free PMC article.
-
Lack of TNF-α-induced MMP-9 production and abnormal E-cadherin redistribution associated with compromised fusion in MCP-1-null macrophages.Am J Pathol. 2011 May;178(5):2311-21. doi: 10.1016/j.ajpath.2011.01.045. Am J Pathol. 2011. PMID: 21514443 Free PMC article.
-
Specific NFkappaB subunit activation and kinetics of cytokine induction in adenoviral keratitis.Mol Vis. 2009 Dec 25;15:2879-89. Mol Vis. 2009. PMID: 20038977 Free PMC article.
-
Targeted disruption of NF-{kappa}B1 (p50) augments cigarette smoke-induced lung inflammation and emphysema in mice: a critical role of p50 in chromatin remodeling.Am J Physiol Lung Cell Mol Physiol. 2010 Feb;298(2):L197-209. doi: 10.1152/ajplung.00265.2009. Epub 2009 Dec 4. Am J Physiol Lung Cell Mol Physiol. 2010. PMID: 19965984 Free PMC article.
-
Cysteinyl Leukotriene Receptor 2 is Involved in Inflammation and Neuronal Damage by Mediating Microglia M1/M2 Polarization through NF-κB Pathway.Neuroscience. 2019 Dec 1;422:99-118. doi: 10.1016/j.neuroscience.2019.10.048. Epub 2019 Nov 11. Neuroscience. 2019. PMID: 31726033
Cited by
-
Different cellular and immunohistochemical abdominal wall cicatrization parameters evaluation in comparison with sublay, onlay, and ipom technique in an experimental rat model.Hernia. 2023 Aug;27(4):999-1015. doi: 10.1007/s10029-023-02740-z. Epub 2023 Jan 18. Hernia. 2023. PMID: 36652036
-
Macrophage and Fibroblast Interactions in Biomaterial-Mediated Fibrosis.Adv Healthc Mater. 2019 Feb;8(4):e1801451. doi: 10.1002/adhm.201801451. Epub 2019 Jan 18. Adv Healthc Mater. 2019. PMID: 30658015 Free PMC article. Review.
-
Collagen Fibril Density Modulates Macrophage Activation and Cellular Functions during Tissue Repair.Bioengineering (Basel). 2020 Mar 31;7(2):33. doi: 10.3390/bioengineering7020033. Bioengineering (Basel). 2020. PMID: 32244521 Free PMC article.
-
Transient Receptor Potential Vanilloid 4 Is Required for Foreign Body Response and Giant Cell Formation.Am J Pathol. 2019 Aug;189(8):1505-1512. doi: 10.1016/j.ajpath.2019.04.016. Epub 2019 May 21. Am J Pathol. 2019. PMID: 31121133 Free PMC article.
-
The innate immune response to ischemic injury: a multiscale modeling perspective.BMC Syst Biol. 2018 Apr 10;12(1):50. doi: 10.1186/s12918-018-0580-z. BMC Syst Biol. 2018. PMID: 29631571 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

