Investigating the efficacy of monovalent and tetravalent dengue vaccine formulations against DENV-4 challenge in AG129 mice
- PMID: 25239488
- PMCID: PMC4252871
- DOI: 10.1016/j.vaccine.2014.08.087
Investigating the efficacy of monovalent and tetravalent dengue vaccine formulations against DENV-4 challenge in AG129 mice
Abstract
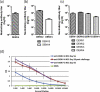
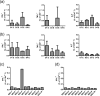
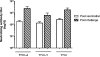
Dengue (DEN) is the most important mosquito-borne viral disease, with a major impact on global health and economics, caused by four serologically and distinct viruses termed DENV-1 to DENV-4. Currently, there is no licensed vaccine to prevent DEN. We have developed a live attenuated tetravalent DENV vaccine candidate (TDV) (formally known as DENVax) that has shown promise in preclinical and clinical studies and elicits neutralizing antibody responses to all four DENVs. As these responses are lowest to DENV-4 we have used the AG129 mouse model to investigate the immunogenicity of monovalent TDV-4 or tetravalent TDV vaccines, and their efficacy against lethal DENV-4 challenge. Since the common backbone of TDV is based on an attenuated DENV-2 strain (TDV-2) we also tested the efficacy of TDV-2 against DENV-4 challenge. Single doses of the tetravalent or monovalent vaccines elicited neutralizing antibodies, anti-NS1 antibodies, and cellular responses to both envelope and nonstructural proteins. All vaccinated animals were protected against challenge at 60 days post-immunization, whereas all control animals died. Investigation of DENV-4 viremias post-challenge showed that only the control animals had high viremias on day 3 post-challenge, whereas vaccinated mice had no detectable viremia. Overall, these data highlight the excellent immunogenicity and efficacy profile of our candidate dengue vaccine in AG129 mice.
Keywords: AG129 mice; Dengue; Dengue 4; Immune protection; Vaccines.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Immunogenicity and efficacy of chimeric dengue vaccine (DENVax) formulations in interferon-deficient AG129 mice.Vaccine. 2012 Feb 14;30(8):1513-20. doi: 10.1016/j.vaccine.2011.11.072. Epub 2011 Dec 13. Vaccine. 2012. PMID: 22178727 Free PMC article.
-
Development of a recombinant, chimeric tetravalent dengue vaccine candidate.Vaccine. 2015 Dec 10;33(50):7112-20. doi: 10.1016/j.vaccine.2015.11.022. Epub 2015 Nov 14. Vaccine. 2015. PMID: 26585500 Review.
-
Improvement of the Dengue Virus (DENV) Nonhuman Primate Model via a Reverse Translational Approach Based on Dengue Vaccine Clinical Efficacy Data against DENV-2 and -4.J Virol. 2018 May 29;92(12):e00440-18. doi: 10.1128/JVI.00440-18. Print 2018 Jun 15. J Virol. 2018. PMID: 29593041 Free PMC article.
-
Immunodomination of Serotype-Specific CD4+ T-Cell Epitopes Contributed to the Biased Immune Responses Induced by a Tetravalent Measles-Vectored Dengue Vaccine.Front Immunol. 2020 Mar 31;11:546. doi: 10.3389/fimmu.2020.00546. eCollection 2020. Front Immunol. 2020. PMID: 32300346 Free PMC article.
-
A recombinant, chimeric tetravalent dengue vaccine candidate based on a dengue virus serotype 2 backbone.Expert Rev Vaccines. 2016;15(4):497-508. doi: 10.1586/14760584.2016.1128328. Epub 2016 Feb 22. Expert Rev Vaccines. 2016. PMID: 26635182 Review.
Cited by
-
Dengue and Zika Virus Domain III-Flagellin Fusion and Glycan-Masking E Antigen for Prime-Boost Immunization.Theranostics. 2019 Jul 9;9(16):4811-4826. doi: 10.7150/thno.35919. eCollection 2019. Theranostics. 2019. PMID: 31367259 Free PMC article.
-
Non-Human Primate Models of Dengue Virus Infection: A Comparison of Viremia Levels and Antibody Responses during Primary and Secondary Infection among Old World and New World Monkeys.Pathogens. 2020 Mar 27;9(4):247. doi: 10.3390/pathogens9040247. Pathogens. 2020. PMID: 32230836 Free PMC article. Review.
-
Mouse models of dengue virus infection for vaccine testing.Vaccine. 2015 Dec 10;33(50):7051-60. doi: 10.1016/j.vaccine.2015.09.112. Epub 2015 Oct 23. Vaccine. 2015. PMID: 26478201 Free PMC article. Review.
-
Zika virus like particles elicit protective antibodies in mice.PLoS Negl Trop Dis. 2018 Feb 5;12(2):e0006210. doi: 10.1371/journal.pntd.0006210. eCollection 2018 Feb. PLoS Negl Trop Dis. 2018. PMID: 29401460 Free PMC article.
-
A Novel, Comprehensive A129 Mouse Model for Investigating Dengue Vaccines and Evaluating Pathogenesis.Vaccines (Basel). 2023 Dec 15;11(12):1857. doi: 10.3390/vaccines11121857. Vaccines (Basel). 2023. PMID: 38140260 Free PMC article.
References
-
- Whitehorn J, Simmons CP. The pathogenesis of dengue. Vaccine. 2011;29:7221–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

