P2Y2R activation by nucleotides released from the highly metastatic breast cancer cell MDA-MB-231 contributes to pre-metastatic niche formation by mediating lysyl oxidase secretion, collagen crosslinking, and monocyte recruitment
- PMID: 25238333
- PMCID: PMC4253437
- DOI: 10.18632/oncotarget.2427
P2Y2R activation by nucleotides released from the highly metastatic breast cancer cell MDA-MB-231 contributes to pre-metastatic niche formation by mediating lysyl oxidase secretion, collagen crosslinking, and monocyte recruitment
Abstract
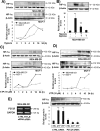
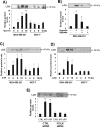
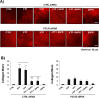
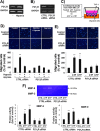
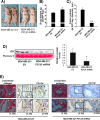
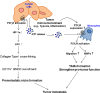
Tumor microenvironmental hypoxia induces hypoxia inducible factor-1α (HIF-1α) overexpression, leading to the release of lysyl oxidase (LOX), which crosslinks collagen at distant sites to facilitate environmental changes that allow cancer cells to easily metastasize. Our previous study showed that activation of the P2Y2 receptor (P2Y2R) by ATP released from MDA-MB-231 cells increased MDA-MB-231 cell invasion through endothelial cells. Therefore, in this study, we investigated the role of P2Y2R in breast cancer cell metastasis to distant sites. ATP or UTP released from hypoxia-treated MDA-MB-231 cells induced HIF-1α expression and LOX secretion by the activation of P2Y2R, and this phenomenon was significantly reduced in P2Y2R-depleted MDA-MB-231 cells. Furthermore, P2Y2R-mediated LOX release induced collagen crosslinking in an in vitro model. Finally, nude mice injected with MDA-MB-231 cells showed high levels of LOX secretion, crosslinked collagen and CD11b+ BMDC recruitment in the lung; however, mice that were injected with P2Y2R-depleted MDA-MB-231 cells did not exhibit these changes. These results demonstrate that P2Y2R plays an important role in activation of the HIF-1α-LOX axis, the induction of collagen crosslinking and the recruitment of CD11b+ BMDCs. Furthermore, P2Y2R activation by nucleotides recruits THP-1 monocytes, resulting in primary tumor progression and pre-metastatic niche formation.
Conflict of interest statement
Authors declare no conflicts of interest.
Figures
Similar articles
-
Radiotherapy-Resistant Breast Cancer Cells Enhance Tumor Progression by Enhancing Premetastatic Niche Formation through the HIF-1α-LOX Axis.Int J Mol Sci. 2020 Oct 28;21(21):8027. doi: 10.3390/ijms21218027. Int J Mol Sci. 2020. PMID: 33126606 Free PMC article.
-
P2Y2 nucleotide receptor-mediated extracellular signal-regulated kinases and protein kinase C activation induces the invasion of highly metastatic breast cancer cells.Oncol Rep. 2015 Jul;34(1):195-202. doi: 10.3892/or.2015.3972. Epub 2015 May 11. Oncol Rep. 2015. PMID: 26063340
-
P2Y2 receptor activation by nucleotides released from highly metastatic breast cancer cells increases tumor growth and invasion via crosstalk with endothelial cells.Breast Cancer Res. 2014 Aug 26;16(5):R77. doi: 10.1186/bcr3694. Breast Cancer Res. 2014. PMID: 25156554 Free PMC article.
-
Lysyl oxidase (LOX) and hypoxia-induced metastases.Cancer Biol Ther. 2006 Aug;5(8):909-11. doi: 10.4161/cbt.5.8.3230. Epub 2006 Aug 26. Cancer Biol Ther. 2006. PMID: 16969095 Review.
-
Lysyl oxidase in cancer inhibition and metastasis.Cancer Lett. 2018 Mar 28;417:174-181. doi: 10.1016/j.canlet.2018.01.006. Epub 2018 Jan 5. Cancer Lett. 2018. PMID: 29309816 Review.
Cited by
-
Sodium Hexametaphosphate Serves as an Inducer of Calcium Signaling.Biomolecules. 2023 Mar 23;13(4):577. doi: 10.3390/biom13040577. Biomolecules. 2023. PMID: 37189325 Free PMC article.
-
Autocrine and paracrine purinergic signaling in the most lethal types of cancer.Purinergic Signal. 2021 Sep;17(3):345-370. doi: 10.1007/s11302-021-09785-8. Epub 2021 May 12. Purinergic Signal. 2021. PMID: 33982134 Free PMC article. Review.
-
P2Y11 receptor is a critical regulator of extracellular ATP-mediated premature senescence in lung fibroblasts: Implications of ER-Ca+2 release/mitochondrial ROS production signaling pathway.Purinergic Signal. 2024 Jul 8. doi: 10.1007/s11302-024-10036-9. Online ahead of print. Purinergic Signal. 2024. PMID: 38977636 No abstract available.
-
Lysyl oxidase engineered lipid nanovesicles for the treatment of triple negative breast cancer.Sci Rep. 2021 Mar 3;11(1):5107. doi: 10.1038/s41598-021-84492-3. Sci Rep. 2021. PMID: 33658580 Free PMC article.
-
P2Y2R-mediated inflammasome activation is involved in tumor progression in breast cancer cells and in radiotherapy-resistant breast cancer.Int J Oncol. 2018 Nov;53(5):1953-1966. doi: 10.3892/ijo.2018.4552. Epub 2018 Sep 4. Int J Oncol. 2018. PMID: 30226596 Free PMC article.
References
-
- Hayes DF, Isaacs C, Stearns V. Prognostic factors in breast cancer: current and new predictors of metastasis. J Mammary Gland Biol Neoplasia. 2001;6:375–392. - PubMed
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
-
- Rugo HS. The importance of distant metastasis in hormone-sensitive breast cancer. Breast. 2008;17:S3–S8. - PubMed
-
- Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. - PubMed
-
- Steeg PS. Cancer: micromanagement of metastasis. Nature. 2007;449:671–673. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

