Filament assembly by Spire: key residues and concerted actin binding
- PMID: 25234086
- PMCID: PMC4324353
- DOI: 10.1016/j.jmb.2014.09.002
Filament assembly by Spire: key residues and concerted actin binding
Abstract
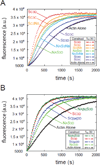
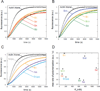
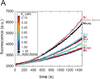
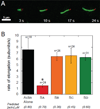
The most recently identified class of actin nucleators, WASp homology domain 2 (WH2) nucleators, use tandem repeats of monomeric actin-binding WH2 domains to facilitate actin nucleation. WH2 domains are involved in a wide variety of actin regulatory activities. Structurally, they are expected to clash with interprotomer contacts within the actin filament. Thus, the discovery of their role in nucleation was surprising. Here we use Drosophila Spire (Spir) as a model system to investigate both how tandem WH2 domains can nucleate actin and what differentiates nucleating WH2-containing proteins from their non-nucleating counterparts. We found that the third WH2 domain in Spir (Spir-C or SC) plays a unique role. In the context of a short nucleation construct (containing only two WH2 domains), placement of SC in the N-terminal position was required for the most potent nucleation. We found that the native organization of the WH2 domains with respect to each other is necessary for binding to actin with positive cooperativity. We identified two residues within SC that are critical for its activity. Using this information, we were able to convert a weak synthetic nucleator into one with activity equal to a native Spir construct. Lastly, we found evidence that SC binds actin filaments, in addition to monomers.
Keywords: Spir; WH2; actin; cytoskeleton; nucleation.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
Multiple forms of Spire-actin complexes and their functional consequences.J Biol Chem. 2012 Mar 23;287(13):10684-10692. doi: 10.1074/jbc.M111.317792. Epub 2012 Feb 8. J Biol Chem. 2012. PMID: 22334675 Free PMC article.
-
Structures of actin-bound Wiskott-Aldrich syndrome protein homology 2 (WH2) domains of Spire and the implication for filament nucleation.Proc Natl Acad Sci U S A. 2010 Jun 29;107(26):11757-62. doi: 10.1073/pnas.1005347107. Epub 2010 Jun 10. Proc Natl Acad Sci U S A. 2010. PMID: 20538977 Free PMC article.
-
Drosophila Spire is an actin nucleation factor.Nature. 2005 Jan 27;433(7024):382-8. doi: 10.1038/nature03241. Nature. 2005. PMID: 15674283
-
The WH2 Domain and Actin Nucleation: Necessary but Insufficient.Trends Biochem Sci. 2016 Jun;41(6):478-490. doi: 10.1016/j.tibs.2016.03.004. Epub 2016 Apr 5. Trends Biochem Sci. 2016. PMID: 27068179 Free PMC article. Review.
-
Control of actin assembly by the WH2 domains and their multifunctional tandem repeats in Spire and Cordon-Bleu.Int Rev Cell Mol Biol. 2011;290:55-85. doi: 10.1016/B978-0-12-386037-8.00005-3. Int Rev Cell Mol Biol. 2011. PMID: 21875562 Review.
Cited by
-
Actin Cross-Linking Toxin Is a Universal Inhibitor of Tandem-Organized and Oligomeric G-Actin Binding Proteins.Curr Biol. 2018 May 21;28(10):1536-1547.e9. doi: 10.1016/j.cub.2018.03.065. Epub 2018 May 3. Curr Biol. 2018. PMID: 29731300 Free PMC article.
-
Drosophila comes of age as a model system for understanding the function of cytoskeletal proteins in cells, tissues, and organisms.Cytoskeleton (Hoboken). 2015 May;72(5):207-24. doi: 10.1002/cm.21228. Epub 2015 Jun 30. Cytoskeleton (Hoboken). 2015. PMID: 26074334 Free PMC article. Review.
-
Structure of a Bud6/Actin Complex Reveals a Novel WH2-like Actin Monomer Recruitment Motif.Structure. 2015 Aug 4;23(8):1492-1499. doi: 10.1016/j.str.2015.05.015. Epub 2015 Jun 25. Structure. 2015. PMID: 26118535 Free PMC article.
-
Phosphorylation of the WH2 domain in yeast Las17/WASP regulates G-actin binding and protein function during endocytosis.Sci Rep. 2021 May 6;11(1):9718. doi: 10.1038/s41598-021-88826-z. Sci Rep. 2021. PMID: 33958621 Free PMC article.
-
Spire stimulates nucleation by Cappuccino and binds both ends of actin filaments.Mol Biol Cell. 2020 Feb 15;31(4):273-286. doi: 10.1091/mbc.E19-09-0550. Epub 2019 Dec 26. Mol Biol Cell. 2020. PMID: 31877067 Free PMC article.
References
-
- Paunola E, Mattila PK, Lappalainen P. WH2 domain: a small, versatile adapter for actin monomers. FEBS Lett. 2002;513:92–97. - PubMed
-
- Carlier MF, Hertzog M, Didry D, Renault L, Cantrelle FX, van Heijenoort C, et al. Structure, function, and evolution of the beta-thymosin/WH2 (WASP-Homology2) actin-binding module. Ann N Acad Sci. 2007;1112:67–75. - PubMed
-
- Husson C, Cantrelle FX, Roblin P, Didry D, Le KH, Perez J, et al. Multifunctionality of the beta-thymosin/WH2 module: G-actin sequestration, actin filament growth, nucleation, and severing. Ann N Acad Sci. 2010;1194:44–52. - PubMed
-
- Qualmann B, Kessels MM. New players in actin polymerization--WH2-domain-containing actin nucleators. Trends Cell Biol. 2009;19:276–285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

