Herpes Simplex Virus 1 (HSV-1) ICP22 protein directly interacts with cyclin-dependent kinase (CDK)9 to inhibit RNA polymerase II transcription elongation
- PMID: 25233083
- PMCID: PMC4169428
- DOI: 10.1371/journal.pone.0107654
Herpes Simplex Virus 1 (HSV-1) ICP22 protein directly interacts with cyclin-dependent kinase (CDK)9 to inhibit RNA polymerase II transcription elongation
Abstract
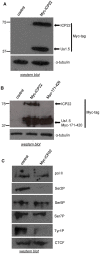
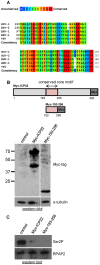
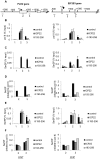
The Herpes Simplex Virus 1 (HSV-1)-encoded ICP22 protein plays an important role in viral infection and affects expression of host cell genes. ICP22 is known to reduce the global level of serine (Ser)2 phosphorylation of the Tyr1Ser2Pro3Thr4Ser5Pro6Ser7 heptapeptide repeats comprising the carboxy-terminal domain (CTD) of the large subunit of RNA polymerase (pol) II. Accordingly, ICP22 is thought to associate with and inhibit the activity of the positive-transcription elongation factor b (P-TEFb) pol II CTD Ser2 kinase. We show here that ICP22 causes loss of CTD Ser2 phosphorylation from pol II engaged in transcription of protein-coding genes following ectopic expression in HeLa cells and that recombinant ICP22 interacts with the CDK9 subunit of recombinant P-TEFb. ICP22 also interacts with pol II in vitro. Residues 193 to 256 of ICP22 are sufficient for interaction with CDK9 and inhibition of pol II CTD Ser2 phosphorylation but do not interact with pol II. These results indicate that discrete regions of ICP22 interact with either CDK9 or pol II and that ICP22 interacts directly with CDK9 to inhibit expression of host cell genes.
Conflict of interest statement
Figures

Similar articles
-
The carboxyl-terminal domain of RNA polymerase II is phosphorylated by a complex containing cdk9 and infected-cell protein 22 of herpes simplex virus 1.J Virol. 2005 Jun;79(11):6757-62. doi: 10.1128/JVI.79.11.6757-6762.2005. J Virol. 2005. PMID: 15890914 Free PMC article.
-
Herpes simplex virus 1 inhibits phosphorylation of RNA polymerase II CTD serine-7.J Virol. 2024 Oct 22;98(10):e0117824. doi: 10.1128/jvi.01178-24. Epub 2024 Sep 24. J Virol. 2024. PMID: 39316591 Free PMC article.
-
Herpes simplex virus 1 ICP22 inhibits the transcription of viral gene promoters by binding to and blocking the recruitment of P-TEFb.PLoS One. 2012;7(9):e45749. doi: 10.1371/journal.pone.0045749. Epub 2012 Sep 24. PLoS One. 2012. PMID: 23029222 Free PMC article.
-
P-TEFb goes viral.Bioessays. 2016 Jul;38 Suppl 1:S75-85. doi: 10.1002/bies.201670912. Bioessays. 2016. PMID: 27417125 Review.
-
RNA polymerase II transcription elongation and Pol II CTD Ser2 phosphorylation: A tail of two kinases.Nucleus. 2014 May-Jun;5(3):224-36. doi: 10.4161/nucl.29347. Epub 2014 May 30. Nucleus. 2014. PMID: 24879308 Free PMC article. Review.
Cited by
-
Cellular state landscape and herpes simplex virus type 1 infection progression are connected.Nat Commun. 2023 Jul 27;14(1):4515. doi: 10.1038/s41467-023-40148-6. Nat Commun. 2023. PMID: 37500668 Free PMC article.
-
Occupancy of RNA Polymerase II Phosphorylated on Serine 5 (RNAP S5P) and RNAP S2P on Varicella-Zoster Virus Genes 9, 51, and 66 Is Independent of Transcript Abundance and Polymerase Location within the Gene.J Virol. 2015 Nov 11;90(3):1231-43. doi: 10.1128/JVI.02617-15. Print 2016 Feb 1. J Virol. 2015. PMID: 26559844 Free PMC article.
-
Cell Culture Evolution of a Herpes Simplex Virus 1 (HSV-1)/Varicella-Zoster Virus (VZV) UL34/ORF24 Chimeric Virus Reveals Novel Functions for HSV Genes in Capsid Nuclear Egress.J Virol. 2021 Nov 9;95(23):e0095721. doi: 10.1128/JVI.00957-21. Epub 2021 Sep 15. J Virol. 2021. PMID: 34523964 Free PMC article.
-
HSV-1 Infection Induces a Downstream Shift of Promoter-Proximal Pausing for Host Genes.J Virol. 2023 May 31;97(5):e0038123. doi: 10.1128/jvi.00381-23. Epub 2023 Apr 24. J Virol. 2023. PMID: 37093003 Free PMC article.
-
The Archer and the Prey: The Duality of PAF1C in Antiviral Immunity.Viruses. 2023 Apr 22;15(5):1032. doi: 10.3390/v15051032. Viruses. 2023. PMID: 37243120 Free PMC article. Review.
References
-
- Liesegang TJ (2001) Herpes simplex virus epidemiology and ocular importance. Cornea 20: 1–13. - PubMed
-
- Knipe DM, Cliffe A (2008) Chromatin control of herpes simplex virus lytic and latent infection. Nat Rev Microbiol 6: 211–221. - PubMed
-
- Galdiero S, Falanga A, Tarallo R, Russo L, Galdiero E, et al. (2013) Peptide inhibitors against herpes simplex virus infections. J Pept Sci 19: 148–158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

