Isoprenoid biosynthesis in Plasmodium falciparum
- PMID: 25217461
- PMCID: PMC4248697
- DOI: 10.1128/EC.00160-14
Isoprenoid biosynthesis in Plasmodium falciparum
Abstract
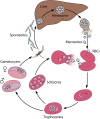
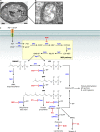
Malaria kills nearly 1 million people each year, and the protozoan parasite Plasmodium falciparum has become increasingly resistant to current therapies. Isoprenoid synthesis via the methylerythritol phosphate (MEP) pathway represents an attractive target for the development of new antimalarials. The phosphonic acid antibiotic fosmidomycin is a specific inhibitor of isoprenoid synthesis and has been a helpful tool to outline the essential functions of isoprenoid biosynthesis in P. falciparum. Isoprenoids are a large, diverse class of hydrocarbons that function in a variety of essential cellular processes in eukaryotes. In P. falciparum, isoprenoids are used for tRNA isopentenylation and protein prenylation, as well as the synthesis of vitamin E, carotenoids, ubiquinone, and dolichols. Recently, isoprenoid synthesis in P. falciparum has been shown to be regulated by a sugar phosphatase. We outline what is known about isoprenoid function and the regulation of isoprenoid synthesis in P. falciparum, in order to identify valuable directions for future research.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
Isoprenoid biosynthesis inhibition disrupts Rab5 localization and food vacuolar integrity in Plasmodium falciparum.Eukaryot Cell. 2013 Feb;12(2):215-23. doi: 10.1128/EC.00073-12. Epub 2012 Dec 7. Eukaryot Cell. 2013. PMID: 23223036 Free PMC article.
-
The methylerythritol phosphate pathway is functionally active in all intraerythrocytic stages of Plasmodium falciparum.J Biol Chem. 2004 Dec 10;279(50):51749-59. doi: 10.1074/jbc.M408360200. Epub 2004 Sep 27. J Biol Chem. 2004. PMID: 15452112
-
A second target of the antimalarial and antibacterial agent fosmidomycin revealed by cellular metabolic profiling.Biochemistry. 2011 May 3;50(17):3570-7. doi: 10.1021/bi200113y. Epub 2011 Apr 11. Biochemistry. 2011. PMID: 21438569 Free PMC article.
-
Fosmidomycin for the treatment of malaria.Parasitol Res. 2003 Jun;90 Suppl 2:S71-6. doi: 10.1007/s00436-002-0770-9. Epub 2002 Nov 30. Parasitol Res. 2003. PMID: 12937969 Review.
-
The isoprenoid-precursor dependence of Plasmodium spp.Nat Prod Rep. 2012 Jul;29(7):721-8. doi: 10.1039/c2np20013a. Epub 2012 May 4. Nat Prod Rep. 2012. PMID: 22555616 Review.
Cited by
-
New insights into apicoplast metabolism in blood-stage malaria parasites.Curr Opin Microbiol. 2023 Feb;71:102255. doi: 10.1016/j.mib.2022.102255. Epub 2022 Dec 21. Curr Opin Microbiol. 2023. PMID: 36563485 Free PMC article. Review.
-
In Vitro Antiplasmodium and Chloroquine Resistance Reversal Effects of Andrographolide.Evid Based Complement Alternat Med. 2019 Dec 13;2019:7967980. doi: 10.1155/2019/7967980. eCollection 2019. Evid Based Complement Alternat Med. 2019. PMID: 31915453 Free PMC article.
-
γδ-T cells promote IFN-γ-dependent Plasmodium pathogenesis upon liver-stage infection.Proc Natl Acad Sci U S A. 2019 May 14;116(20):9979-9988. doi: 10.1073/pnas.1814440116. Epub 2019 Apr 26. Proc Natl Acad Sci U S A. 2019. PMID: 31028144 Free PMC article.
-
A mevalonate bypass system facilitates elucidation of plastid biology in malaria parasites.PLoS Pathog. 2020 Feb 14;16(2):e1008316. doi: 10.1371/journal.ppat.1008316. eCollection 2020 Feb. PLoS Pathog. 2020. PMID: 32059044 Free PMC article.
-
GAPDH mediates drug resistance and metabolism in Plasmodium falciparum malaria parasites.PLoS Pathog. 2022 Sep 14;18(9):e1010803. doi: 10.1371/journal.ppat.1010803. eCollection 2022 Sep. PLoS Pathog. 2022. PMID: 36103572 Free PMC article.
References
-
- Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat DWN. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 455–467. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

