Suppression of miR-184 in malignant gliomas upregulates SND1 and promotes tumor aggressiveness
- PMID: 25216670
- PMCID: PMC4483100
- DOI: 10.1093/neuonc/nou220
Suppression of miR-184 in malignant gliomas upregulates SND1 and promotes tumor aggressiveness
Abstract
Background: Malignant glioma is an aggressive cancer requiring new therapeutic targets. MicroRNAs (miRNAs) regulate gene expression post transcriptionally and are implicated in cancer development and progression. Deregulated expressions of several miRNAs, specifically hsa-miR-184, correlate with glioma development.
Methods: Bioinformatic approaches were used to identify potential miR-184-regulated target genes involved in malignant glioma progression. This strategy identified a multifunctional nuclease, SND1, known to be overexpressed in multiple cancers, including breast, colon, and hepatocellular carcinoma, as a putative direct miR-184 target gene. SND1 levels were evaluated in patient tumor samples and human-derived cell lines. We analyzed invasion and signaling in vitro through SND1 gain-of-function and loss-of-function. An orthotopic xenograft model with primary glioma cells demonstrated a role of miR-184/SND1 in glioma pathogenesis in vivo.
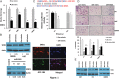
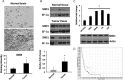
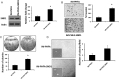
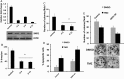
Results: SND1 is highly expressed in human glioma tissue and inversely correlated with miR-184 expression. Transfection of glioma cells with a miR-184 mimic inhibited invasion, suppressed colony formation, and reduced anchorage-independent growth in soft agar. Similar phenotypes were evident when SND1 was knocked down with siRNA. Additionally, knockdown (KD) of SND1 induced senescence and improved the chemoresistant properties of malignant glioma cells. In an orthotopic xenograft model, KD of SND1 or transfection with a miR-184 mimic induced a less invasive tumor phenotype and significantly improved survival of tumor bearing mice.
Conclusions: Our study is the first to show a novel regulatory role of SND1, a direct target of miR-184, in glioma progression, suggesting that the miR-184/SND1 axis may be a useful diagnostic and therapeutic tool for malignant glioma.
Keywords: SND1; intracranial injection; invasion; malignant glioma; miR-184.
© The Author(s) 2014. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
miR-320a functions as a suppressor for gliomas by targeting SND1 and β-catenin, and predicts the prognosis of patients.Oncotarget. 2017 Mar 21;8(12):19723-19737. doi: 10.18632/oncotarget.14975. Oncotarget. 2017. PMID: 28160566 Free PMC article.
-
The novel chromatin architectural regulator SND1 promotes glioma proliferation and invasion and predicts the prognosis of patients.Neuro Oncol. 2019 Jun 10;21(6):742-754. doi: 10.1093/neuonc/noz038. Neuro Oncol. 2019. PMID: 30753603 Free PMC article.
-
miR-548b inhibits the proliferation and invasion of malignant gliomas by targeting metastasis tumor-associated protein-2.Neuroreport. 2016 Dec 7;27(17):1266-1273. doi: 10.1097/WNR.0000000000000690. Neuroreport. 2016. PMID: 27682888
-
The role of microRNAs in glioma initiation and progression.Front Biosci (Landmark Ed). 2012 Jan 1;17(2):700-12. doi: 10.2741/3952. Front Biosci (Landmark Ed). 2012. PMID: 22201769 Free PMC article. Review.
-
Natural products in suppressing glioma progression: A focus on the role of microRNAs.Phytother Res. 2022 Apr;36(4):1576-1599. doi: 10.1002/ptr.7414. Epub 2022 Feb 16. Phytother Res. 2022. PMID: 35174549 Review.
Cited by
-
Inhibitory effect of miR-184 on the potential of proliferation and invasion in human glioma and breast cancer cells in vitro.Int J Clin Exp Pathol. 2015 Aug 1;8(8):9376-82. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26464691 Free PMC article.
-
Insights Into SND1 Oncogene Promoter Regulation.Front Oncol. 2018 Dec 11;8:606. doi: 10.3389/fonc.2018.00606. eCollection 2018. Front Oncol. 2018. PMID: 30619748 Free PMC article. Review.
-
Tumor Suppressor miR-184 Enhances Chemosensitivity by Directly Inhibiting SLC7A5 in Retinoblastoma.Front Oncol. 2019 Nov 15;9:1163. doi: 10.3389/fonc.2019.01163. eCollection 2019. Front Oncol. 2019. PMID: 31803607 Free PMC article.
-
miR-361-5p inhibits glioma migration and invasion by targeting SND1.Onco Targets Ther. 2018 Aug 28;11:5239-5252. doi: 10.2147/OTT.S171539. eCollection 2018. Onco Targets Ther. 2018. PMID: 30214229 Free PMC article.
-
HAND2-AS1 Inhibits Gastric Adenocarcinoma Cells Proliferation and Aerobic Glycolysis via miRNAs Sponge.Cancer Manag Res. 2020 May 1;12:3053-3068. doi: 10.2147/CMAR.S222878. eCollection 2020. Cancer Manag Res. 2020. PMID: 32431548 Free PMC article.
References
-
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. - PubMed
-
- Lefranc F, Brotchi J, Kiss R. Possible future issues in the treatment of glioblastomas: special emphasis on cell migration and the resistance of migrating glioblastoma cells to apoptosis. J Clin Oncol. 2005;23(10):2411–2422. - PubMed
-
- Schickel R, Boyerinas B, Park SM, et al. MicroRNAs: key players in the immune system, differentiation, tumorigenesis and cell death. Oncogene. 2008;27(45):5959–5974. - PubMed
-
- Chen CZ. MicroRNAs as oncogenes and tumor suppressors. N Engl J Med. 2005;353(17):1768–1771. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

