Spatial control of Cdc42 signalling by a GM130-RasGRF complex regulates polarity and tumorigenesis
- PMID: 25208761
- PMCID: PMC4449154
- DOI: 10.1038/ncomms5839
Spatial control of Cdc42 signalling by a GM130-RasGRF complex regulates polarity and tumorigenesis
Abstract
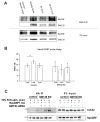
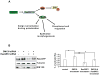
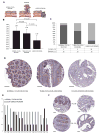
The small GTPase Cdc42 is a key regulator of polarity, but little is known in mammals about its spatial regulation and the relevance of spatial Cdc42 pools for polarity. Here we report the identification of a GM130-RasGRF complex as a regulator of Cdc42 at the Golgi. Silencing GM130 results in RasGRF-dependent inhibition of the Golgi pool of Cdc42, but does not affect Cdc42 at the cell surface. Furthermore, active Cdc42 at the Golgi is important to sustain asymmetric front-rear Cdc42-GTP distribution in directionally migrating cells. Concurrent to Cdc42 inhibition, silencing GM130 also results in RasGRF-dependent Ras-ERK pathway activation. Moreover, depletion of GM130 is sufficient to induce E-cadherin downregulation, indicative of a loss in cell polarity and epithelial identity. Accordingly, GM130 expression is frequently lost in colorectal and breast cancer patients. These findings establish a previously unrecognized role for a GM130-RasGRF-Cdc42 connection in regulating polarity and tumorigenesis.
Conflict of interest statement
The authors declare that no competing financial interests exist.
Figures
Similar articles
-
Endomembrane control of cell polarity: Relevance to cancer.Small GTPases. 2015;6(2):104-7. doi: 10.1080/21541248.2015.1018402. Small GTPases. 2015. PMID: 26156751 Free PMC article. Review.
-
Loss of GM130 in breast cancer cells and its effects on cell migration, invasion and polarity.Cell Cycle. 2015;14(8):1139-47. doi: 10.1080/15384101.2015.1007771. Cell Cycle. 2015. PMID: 25892554 Free PMC article.
-
Structural and spatial determinants regulating TC21 activation by RasGRF family nucleotide exchange factors.Mol Biol Cell. 2009 Oct;20(20):4289-302. doi: 10.1091/mbc.e09-03-0212. Epub 2009 Aug 19. Mol Biol Cell. 2009. PMID: 19692568 Free PMC article.
-
GM130-dependent control of Cdc42 activity at the Golgi regulates centrosome organization.Mol Biol Cell. 2009 Feb;20(4):1192-200. doi: 10.1091/mbc.e08-08-0834. Epub 2008 Dec 24. Mol Biol Cell. 2009. PMID: 19109421 Free PMC article.
-
Cdc42 in oncogenic transformation, invasion, and tumorigenesis.Cell Signal. 2011 Sep;23(9):1415-23. doi: 10.1016/j.cellsig.2011.04.001. Epub 2011 Apr 16. Cell Signal. 2011. PMID: 21515363 Free PMC article. Review.
Cited by
-
Spatiotemporal Control of Intracellular Membrane Trafficking by Rho GTPases.Cells. 2019 Nov 21;8(12):1478. doi: 10.3390/cells8121478. Cells. 2019. PMID: 31766364 Free PMC article. Review.
-
Activation of ULK Kinase and Autophagy by GABARAP Trafficking from the Centrosome Is Regulated by WAC and GM130.Mol Cell. 2015 Dec 17;60(6):899-913. doi: 10.1016/j.molcel.2015.11.018. Mol Cell. 2015. PMID: 26687599 Free PMC article.
-
Alterations of Golgi Structural Proteins and Glycosylation Defects in Cancer.Front Cell Dev Biol. 2021 May 12;9:665289. doi: 10.3389/fcell.2021.665289. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34055798 Free PMC article. Review.
-
Actin remodeling by Nck regulates endothelial lumen formation.Mol Biol Cell. 2015 Sep 1;26(17):3047-60. doi: 10.1091/mbc.E15-06-0338. Epub 2015 Jul 8. Mol Biol Cell. 2015. PMID: 26157164 Free PMC article.
-
Alteration of Golgi Structure by Stress: A Link to Neurodegeneration?Front Neurosci. 2015 Nov 12;9:435. doi: 10.3389/fnins.2015.00435. eCollection 2015. Front Neurosci. 2015. PMID: 26617486 Free PMC article.
References
-
- Etienne-Manneville S. Cdc42 - the centre of polarity. J Cell Sci. 2004;117:1291–1300. - PubMed
-
- Iden S, et al. Tumor Type-Dependent Function of the Par3 Polarity Protein in Skin Tumorigenesis. Cancer Cell. 2012;22:389–403. - PubMed
-
- Erickson JW, Zhang C-j, Kahn RA, Evans T, Cerione RA. Mammalian Cdc42 Is a Brefeldin A-sensitive Component of the Golgi Apparatus. Journal of Biological Chemistry. 1996;271:26850–26854. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

