Intraspinal transplantation of motoneuron-like cell combined with delivery of polymer-based glial cell line-derived neurotrophic factor for repair of spinal cord contusion injury
- PMID: 25206752
- PMCID: PMC4146307
- DOI: 10.4103/1673-5374.133159
Intraspinal transplantation of motoneuron-like cell combined with delivery of polymer-based glial cell line-derived neurotrophic factor for repair of spinal cord contusion injury
Abstract
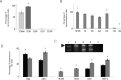
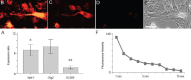
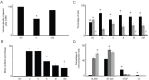
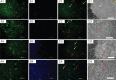
To evaluate the effects of glial cell line-derived neurotrophic factor transplantation combined with adipose-derived stem cells-transdifferentiated motoneuron delivery on spinal cord contusion injury, we developed rat models of spinal cord contusion injury, 7 days later, injected adipose-derived stem cells-transdifferentiated motoneurons into the epicenter, rostral and caudal regions of the impact site and simultaneously transplanted glial cell line-derived neurotrophic factor-gelfoam complex into the myelin sheath. Motoneuron-like cell transplantation combined with glial cell line-derived neurotrophic factor delivery reduced cavity formations and increased cell density in the transplantation site. The combined therapy exhibited superior promoting effects on recovery of motor function to transplantation of glial cell line-derived neurotrophic factor, adipose-derived stem cells or motoneurons alone. These findings suggest that motoneuron-like cell transplantation combined with glial cell line-derived neurotrophic factor delivery holds a great promise for repair of spinal cord injury.
Keywords: adipose-derived stem cells; cell transplantation; glial cell line-derived neurotrophic factor; motoneurons; nerve regeneration; neural regeneration; neurotrophic factor; spinal cord contusion injury; spinal cord injury.
Conflict of interest statement
Figures


Similar articles
-
Rescue and sprouting of motoneurons following ventral root avulsion and reimplantation combined with intraspinal adeno-associated viral vector-mediated expression of glial cell line-derived neurotrophic factor or brain-derived neurotrophic factor.Exp Neurol. 2004 Oct;189(2):303-16. doi: 10.1016/j.expneurol.2004.05.014. Exp Neurol. 2004. PMID: 15380481
-
[Effects of embryonic neural stem cells and glial cell line-derived neurotrophic factor in the repair of spinal cord injury].Sheng Li Xue Bao. 2003 Jun 25;55(3):349-54. Sheng Li Xue Bao. 2003. PMID: 12817305 Chinese.
-
Combined transplantation of GDAs(BMP) and hr-decorin in spinal cord contusion repair.Neural Regen Res. 2013 Aug 25;8(24):2236-48. doi: 10.3969/j.issn.1673-5374.2013.24.003. Neural Regen Res. 2013. PMID: 25206533 Free PMC article.
-
Neurotrophic factors for spinal cord repair: Which, where, how and when to apply, and for what period of time?Brain Res. 2015 Sep 4;1619:36-71. doi: 10.1016/j.brainres.2014.10.049. Epub 2014 Nov 1. Brain Res. 2015. PMID: 25451132 Review.
-
Adenoviral gene transfer of glial cell line-derived neurotrophic factor to injured adult motoneurons.Hum Cell. 2001 Mar;14(1):7-15. Hum Cell. 2001. PMID: 11436355 Review.
Cited by
-
Trans-Differentiation of Human Dental Pulp Stem Cells Into Cholinergic-Like Neurons Via Nerve Growth Factor.Basic Clin Neurosci. 2019 Nov-Dec;10(6):609-617. doi: 10.32598/bcn.10.6.609. Epub 2019 Nov 1. Basic Clin Neurosci. 2019. PMID: 32477478 Free PMC article.
-
Molecular Mechanisms in the Vascular and Nervous Systems following Traumatic Spinal Cord Injury.Life (Basel). 2022 Dec 20;13(1):9. doi: 10.3390/life13010009. Life (Basel). 2022. PMID: 36675958 Free PMC article. Review.
-
Efficacy of adipose tissue-derived stem cells in locomotion recovery after spinal cord injury: a systematic review and meta-analysis on animal studies.Syst Rev. 2021 Jul 31;10(1):213. doi: 10.1186/s13643-021-01771-w. Syst Rev. 2021. PMID: 34330329 Free PMC article. Review.
-
Intrathecal delivery of adipose-derived mesenchymal stem cells in traumatic spinal cord injury: Phase I trial.Nat Commun. 2024 Apr 1;15(1):2201. doi: 10.1038/s41467-024-46259-y. Nat Commun. 2024. PMID: 38561341 Free PMC article. Clinical Trial.
-
Creation of an intramedullary cavity by hemorrhagic necrosis removal 24 h after spinal cord contusion in rats for eventual intralesional implantation of restorative materials.PLoS One. 2017 Apr 17;12(4):e0176105. doi: 10.1371/journal.pone.0176105. eCollection 2017. PLoS One. 2017. PMID: 28414769 Free PMC article.
References
-
- Abdanipour A, Tiraihi T. Induction of adipose-derived stem cell into motoneuron-like cells using selegiline as preinducer. Brain Res. 2012;1440:23–33. - PubMed
-
- Alexanian AR, Crowe MJ, Kurpad SN. Efficient differentiation and integration of lineage-restricted neural precursors in the traumatically injured adult cat spinal cord. J Neurosci Methods. 2006;150:41–46. - PubMed
-
- Arboleda D, Forostyak S, Jendelova P, Marekova D, Amemori T, Pivonkova H, Masinova K, Sykova E. Transplantation of predifferentiated adipose-derived stromal cells for the treatment of spinal cord injury. Cell Mol Neurobiol. 2011;31:1113–1122. - PubMed
-
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
