Phage display creates innovative applications to combat hepatitis B virus
- PMID: 25206271
- PMCID: PMC4155357
- DOI: 10.3748/wjg.v20.i33.11650
Phage display creates innovative applications to combat hepatitis B virus
Abstract
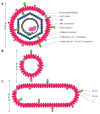
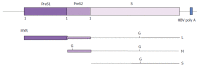
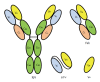
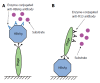
Hepatitis B virus (HBV) has killed countless lives in human history. The invention of HBV vaccines in the 20(th) century has reduced significantly the rate of the viral infection. However, currently there is no effective treatment for chronic HBV carriers. Newly emerging vaccine escape mutants and drug resistant strains have complicated the viral eradication program. The entire world is now facing a new threat of HBV and human immunodeficiency virus co-infection. Could phage display provide solutions to these life-threatening problems? This article reviews critically and comprehensively the innovative and potential applications of phage display in the development of vaccines, therapeutic agents, diagnostic reagents, as well as gene and drug delivery systems to combat HBV. The application of phage display in epitope mapping of HBV antigens is also discussed in detail. Although this review mainly focuses on HBV, the innovative applications of phage display could also be extended to other infectious diseases.
Keywords: Antiviral drug; Diagnosis; Drug delivery; Epitope mapping; Gene delivery; Hepatitis B virus; Hepatocellular carcinoma; Phage display; Therapeutics; Vaccine; Virus-like particle.
Figures

Similar articles
-
Bacteriophages and their applications in the diagnosis and treatment of hepatitis B virus infection.World J Gastroenterol. 2014 Sep 7;20(33):11671-83. doi: 10.3748/wjg.v20.i33.11671. World J Gastroenterol. 2014. PMID: 25206272 Free PMC article. Review.
-
Applications of human hepatitis B virus preS domain in bio- and nanotechnology.World J Gastroenterol. 2015 Jun 28;21(24):7400-11. doi: 10.3748/wjg.v21.i24.7400. World J Gastroenterol. 2015. PMID: 26139986 Free PMC article. Review.
-
Update on hepatitis B virus infection.World J Gastroenterol. 2014 Oct 7;20(37):13293-305. doi: 10.3748/wjg.v20.i37.13293. World J Gastroenterol. 2014. PMID: 25309066 Free PMC article. Review.
-
Hepatitis B virus therapy: What's the future holding for us?World J Gastroenterol. 2015 Nov 28;21(44):12558-75. doi: 10.3748/wjg.v21.i44.12558. World J Gastroenterol. 2015. PMID: 26640332 Free PMC article. Review.
-
Clinical impact of hepatitis B and C virus envelope glycoproteins.World J Gastroenterol. 2013 Feb 7;19(5):654-64. doi: 10.3748/wjg.v19.i5.654. World J Gastroenterol. 2013. PMID: 23429668 Free PMC article. Review.
Cited by
-
Chimeric Virus-Like Particles of Prawn Nodavirus Displaying Hepatitis B Virus Immunodominant Region: Biophysical Properties and Cytokine Response.Int J Mol Sci. 2021 Feb 15;22(4):1922. doi: 10.3390/ijms22041922. Int J Mol Sci. 2021. PMID: 33672018 Free PMC article.
-
HSP60 mimetic peptides from Mycobacterium leprae as new antigens for immunodiagnosis of Leprosy.AMB Express. 2023 Oct 27;13(1):120. doi: 10.1186/s13568-023-01625-9. AMB Express. 2023. PMID: 37891336 Free PMC article.
-
Phage-displayed peptides that mimic epitopes of hepatitis E virus capsid.Med Microbiol Immunol. 2017 Aug;206(4):301-309. doi: 10.1007/s00430-017-0507-0. Epub 2017 Apr 22. Med Microbiol Immunol. 2017. PMID: 28434129
-
Virus like particles as a platform for cancer vaccine development.PeerJ. 2017 Nov 15;5:e4053. doi: 10.7717/peerj.4053. eCollection 2017. PeerJ. 2017. PMID: 29158984 Free PMC article.
-
Immunological Analysis of the Hepatitis B Virus "a" Determinant Displayed on Chimeric Virus-Like Particles of Macrobrachium rosenbergii Nodavirus Capsid Protein Produced in Sf9 Cells.Vaccines (Basel). 2020 Jun 4;8(2):275. doi: 10.3390/vaccines8020275. Vaccines (Basel). 2020. PMID: 32512923 Free PMC article.
References
-
- Michel ML, Tiollais P. Hepatitis B vaccines: protective efficacy and therapeutic potential. Pathol Biol (Paris) 2010;58:288–295. - PubMed
-
- Dane DS, Cameron CH, Briggs M. Virus-like particles in serum of patients with Australia-antigen-associated hepatitis. Lancet. 1970;1:695–698. - PubMed
-
- Bruss V. Envelopment of the hepatitis B virus nucleocapsid. Virus Res. 2004;106:199–209. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

