Antibodies to the extracellular pore loop of TRPM8 act as antagonists of channel activation
- PMID: 25203266
- PMCID: PMC4159296
- DOI: 10.1371/journal.pone.0107151
Antibodies to the extracellular pore loop of TRPM8 act as antagonists of channel activation
Abstract
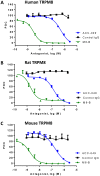
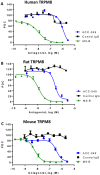
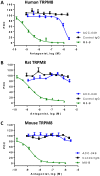
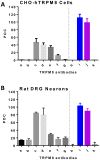
The mammalian transient receptor potential melastatin channel 8 (TRPM8) is highly expressed in trigeminal and dorsal root ganglia. TRPM8 is activated by cold temperature or compounds that cause a cooling sensation, such as menthol or icilin. TRPM8 may play a role in cold hypersensitivity and hyperalgesia in various pain syndromes. Therefore, TRPM8 antagonists are pursued as therapeutics. In this study we explored the feasibility of blocking TRPM8 activation with antibodies. We report the functional characterization of a rabbit polyclonal antibody, ACC-049, directed against the third extracellular loop near the pore region of the human TRPM8 channel. ACC-049 acted as a full antagonist at recombinantly expressed human and rodent TRPM8 channels in cell based agonist-induced 45Ca2+ uptake assays. Further, several poly-and monoclonal antibodies that recognize the same region also blocked icilin activation of not only recombinantly expressed TRPM8, but also endogenous TRPM8 expressed in rat dorsal root ganglion neurons revealing the feasibility of generating monoclonal antibody antagonists. We conclude that antagonist antibodies are valuable tools to investigate TRPM8 function and may ultimately pave the way for development of therapeutic antibodies.
Conflict of interest statement
Figures


Similar articles
-
TRPM8 and Migraine.Headache. 2016 Oct;56(9):1406-1417. doi: 10.1111/head.12948. Epub 2016 Sep 16. Headache. 2016. PMID: 27634619 Free PMC article. Review.
-
P2Y1 Purinergic Receptor Contributes to Remifentanil-Induced Cold Hyperalgesia via Transient Receptor Potential Melastatin 8-Dependent Regulation of N-methyl-d-aspartate Receptor Phosphorylation in Dorsal Root Ganglion.Anesth Analg. 2021 Sep 1;133(3):794-810. doi: 10.1213/ANE.0000000000005617. Anesth Analg. 2021. PMID: 34166321
-
Effects of antagonists and heat on TRPM8 channel currents in dorsal root ganglion neuron activated by nociceptive cold stress and menthol.Neurochem Res. 2012 Feb;37(2):314-20. doi: 10.1007/s11064-011-0614-z. Epub 2011 Oct 1. Neurochem Res. 2012. PMID: 21964764
-
Camphor activates and sensitizes transient receptor potential melastatin 8 (TRPM8) to cooling and icilin.Chem Senses. 2013 Sep;38(7):563-75. doi: 10.1093/chemse/bjt027. Epub 2013 Jul 4. Chem Senses. 2013. PMID: 23828908
-
The emerging pharmacology of TRPM8 channels: hidden therapeutic potential underneath a cold surface.Curr Pharm Biotechnol. 2011 Jan 1;12(1):54-67. doi: 10.2174/138920111793937916. Curr Pharm Biotechnol. 2011. PMID: 20932258 Review.
Cited by
-
Transient Receptor Potential (TRP) Ion Channels Involved in Malignant Glioma Cell Death and Therapeutic Perspectives.Front Cell Dev Biol. 2021 Aug 12;9:618961. doi: 10.3389/fcell.2021.618961. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34458247 Free PMC article. Review.
-
Development of TRPM8 Antagonists to Treat Chronic Pain and Migraine.Pharmaceuticals (Basel). 2017 Mar 30;10(2):37. doi: 10.3390/ph10020037. Pharmaceuticals (Basel). 2017. PMID: 28358322 Free PMC article. Review.
-
TRPM8 and Migraine.Headache. 2016 Oct;56(9):1406-1417. doi: 10.1111/head.12948. Epub 2016 Sep 16. Headache. 2016. PMID: 27634619 Free PMC article. Review.
-
TRPM8 is required for survival and radioresistance of glioblastoma cells.Oncotarget. 2017 Sep 30;8(56):95896-95913. doi: 10.18632/oncotarget.21436. eCollection 2017 Nov 10. Oncotarget. 2017. PMID: 29221175 Free PMC article.
-
Heat Sensing Receptor TRPV1 Is a Mediator of Thermotaxis in Human Spermatozoa.PLoS One. 2016 Dec 16;11(12):e0167622. doi: 10.1371/journal.pone.0167622. eCollection 2016. PLoS One. 2016. PMID: 27992447 Free PMC article.
References
-
- McKemy DD, Neuhausser WM, Julius D (2002) Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416: 52–58. - PubMed
-
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, et al. (2002) A TRP channel that senses cold stimuli and menthol. Cell 108: 705–715. - PubMed
-
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, et al. (2007) The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448: 204–208. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

