Regulation of the tumor suppressor FOXO3 by the thromboxane-A2 receptors in urothelial cancer
- PMID: 25202904
- PMCID: PMC4159332
- DOI: 10.1371/journal.pone.0107530
Regulation of the tumor suppressor FOXO3 by the thromboxane-A2 receptors in urothelial cancer
Abstract
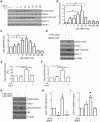
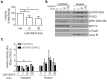
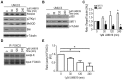
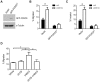
The transcription factor FOXO3 is a well-established tumor suppressor whose activity, stability, and localization are regulated by phosphorylation and acetylation. Previous data by our laboratory demonstrated amplified thromboxane-A2 signaling was associated with poor prognoses in bladder cancer patients and overexpression of the thromboxane-A2 isoform-β receptor (TPβ), but not TPα, induced malignant transformation of immortalized bladder cells in vivo. Here, we describe a mechanism of TP mediated modulation of FOXO3 activity and localization by phosphorylation and deacetylation in a bladder cancer cell model. In vitro gain and loss of function studies performed in non-transformed cell lines, UROsta and SV-HUC, revealed knockdown of FOXO3 expression by shRNA increased cell migration and invasion, while exogenously overexpressing TPβ raised basal phosphorylated (p)FOXO3-S294 levels. Conversely, overexpression of ERK-resistant, mutant FOXO3 reduced increases in UMUC3 cell migration and invasion, including that mediated by TP agonist (U46619). Additionally, stimulation of UMUC3 cells with U46619 increased pFOXO3-S294 expression, which could be attenuated by treatment with a TP antagonist (PTXA2) or ERK inhibitor (U0126). Initially U46619 caused nuclear accumulation of pFOXO3-S294; however, prolonged stimulation increased FOXO3 cytoplasmic localization. U46619 stimulation decreased overall FOXO3 transcriptional activity, but was associated with increased expression of its pro-survival target, manganese superoxide dismutase. The data also shows that TP stimulation increased the expression of the histone deacetylase, SIRT1, and corresponded with decreased acetylated-FOXO3. Collectively, the data suggest a role for TP signaling in the regulation of FOXO3 activity, mediated in part through phosphorylation and deacetylation.
Conflict of interest statement
Figures

Similar articles
-
Different pathways for activation of extracellular signal-regulated kinase through thromboxane A2 receptor isoforms.Biol Pharm Bull. 2006 Apr;29(4):719-24. doi: 10.1248/bpb.29.719. Biol Pharm Bull. 2006. PMID: 16595906
-
Homologous desensitization of signalling by the beta (beta) isoform of the human thromboxane A2 receptor.Biochim Biophys Acta. 2006 Sep;1761(9):1114-31. doi: 10.1016/j.bbalip.2006.07.012. Epub 2006 Aug 3. Biochim Biophys Acta. 2006. PMID: 16956790
-
Regulation of protein kinase C-related kinase (PRK) signalling by the TPα and TPβ isoforms of the human thromboxane A2 receptor: Implications for thromboxane- and androgen- dependent neoplastic and epigenetic responses in prostate cancer.Biochim Biophys Acta Mol Basis Dis. 2017 Apr;1863(4):838-856. doi: 10.1016/j.bbadis.2017.01.011. Epub 2017 Jan 18. Biochim Biophys Acta Mol Basis Dis. 2017. PMID: 28108419
-
Preparing to strike: Acute events in signaling by the serpentine receptor for thromboxane A2.Pharmacol Ther. 2023 Aug;248:108478. doi: 10.1016/j.pharmthera.2023.108478. Epub 2023 Jun 13. Pharmacol Ther. 2023. PMID: 37321373 Review.
-
The rules and regulatory mechanisms of FOXO3 on inflammation, metabolism, cell death and aging in hosts.Life Sci. 2023 Sep 1;328:121877. doi: 10.1016/j.lfs.2023.121877. Epub 2023 Jun 22. Life Sci. 2023. PMID: 37352918 Review.
Cited by
-
A Peroxidase Peroxiredoxin 1-Specific Redox Regulation of the Novel FOXO3 microRNA Target let-7.Antioxid Redox Signal. 2018 Jan 1;28(1):62-77. doi: 10.1089/ars.2016.6871. Epub 2017 May 1. Antioxid Redox Signal. 2018. PMID: 28398822 Free PMC article.
-
miR-93 promotes cell proliferation in gliomas through activation of PI3K/Akt signaling pathway.Oncotarget. 2015 Apr 10;6(10):8286-99. doi: 10.18632/oncotarget.3221. Oncotarget. 2015. PMID: 25823655 Free PMC article.
-
Knockdown of SIRT1 Suppresses Bladder Cancer Cell Proliferation and Migration and Induces Cell Cycle Arrest and Antioxidant Response through FOXO3a-Mediated Pathways.Biomed Res Int. 2017;2017:3781904. doi: 10.1155/2017/3781904. Epub 2017 Sep 25. Biomed Res Int. 2017. PMID: 29147649 Free PMC article.
-
Thromboxane A2 receptor antagonist SQ29548 reduces ischemic stroke-induced microglia/macrophages activation and enrichment, and ameliorates brain injury.Sci Rep. 2016 Oct 24;6:35885. doi: 10.1038/srep35885. Sci Rep. 2016. PMID: 27775054 Free PMC article.
-
Role of Nurr1 in Carcinogenesis and Tumor Immunology: A State of the Art Review.Cancers (Basel). 2020 Oct 19;12(10):3044. doi: 10.3390/cancers12103044. Cancers (Basel). 2020. PMID: 33086676 Free PMC article. Review.
References
-
- Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA: a cancer journal for clinicians 62: 10–29. - PubMed
-
- de Braud F, Maffezzini M, Vitale V, Bruzzi P, Gatta G, et al. (2002) Bladder cancer. Crit Rev Oncol Hematol 41: 89–106. - PubMed
-
- Moussa O, Yordy JS, Abol-Enein H, Sinha D, Bissada NK, et al. (2005) Prognostic and functional significance of thromboxane synthase gene overexpression in invasive bladder cancer. Cancer research 65: 11581–11587. - PubMed
-
- Needleman P, Minkes M, Raz A (1976) Thromboxanes: selective biosynthesis and distinct biological properties. Science 193: 163–165. - PubMed
-
- Sakai H, Suzuki T, Takahashi Y, Ukai M, Tauchi K, et al. (2006) Upregulation of thromboxane synthase in human colorectal carcinoma and the cancer cell proliferation by thromboxane A2. FEBS letters 580: 3368–3374. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

