Preferential infection of human Ad5-specific CD4 T cells by HIV in Ad5 naturally exposed and recombinant Ad5-HIV vaccinated individuals
- PMID: 25197078
- PMCID: PMC4169982
- DOI: 10.1073/pnas.1400446111
Preferential infection of human Ad5-specific CD4 T cells by HIV in Ad5 naturally exposed and recombinant Ad5-HIV vaccinated individuals
Abstract
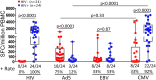
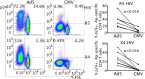
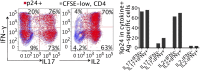
Efficacy trials of adenovirus 5-vectored candidate HIV vaccines [recombinant Ad5 (rAd5)-HIV] were halted for futility due to lack of vaccine efficacy and unexpected excess HIV infections in the vaccine recipients. The potential immunologic basis for these observations is unclear. We comparatively evaluated the HIV susceptibility and phenotypes of human CD4 T cells specific to Ad5 and CMV, two viruses that have been used as HIV vaccine vectors. We show that Ad5-specific CD4 T cells, either induced by natural Ad5 exposure or expanded by rAd5 vaccination, are highly susceptible to HIV in vitro and are preferentially lost in HIV-infected individuals compared with CMV-specific CD4 T cells. Further investigation demonstrated that Ad5-specific CD4 T cells selectively display a proinflammatory Th17-like phenotype and express macrophage inflammatory protein 3α and α4β7 integrin, suggestive of gut mucosa homing potential of these cells. Analysis of HIV p24 and cytokine coexpression using flow cytometry revealed preferential infection of IL-17- and IL-2-producing, Ad5-specific CD4 T cells by HIV in vitro. Our data suggest a potential mechanism explaining the excess HIV infections in vaccine recipients after rAd5-HIV vaccination and highlight the importance of testing the HIV susceptibility of vaccine-generated, vector and insert-specific CD4 T cells in future HIV vaccine studies.
Keywords: AIDS; antigen-specific CD4 T cells; viral vectors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Distinct susceptibility of HIV vaccine vector-induced CD4 T cells to HIV infection.PLoS Pathog. 2018 Feb 23;14(2):e1006888. doi: 10.1371/journal.ppat.1006888. eCollection 2018 Feb. PLoS Pathog. 2018. PMID: 29474461 Free PMC article.
-
Adenovirus vector vaccination induces expansion of memory CD4 T cells with a mucosal homing phenotype that are readily susceptible to HIV-1.Proc Natl Acad Sci U S A. 2009 Nov 24;106(47):19940-5. doi: 10.1073/pnas.0907898106. Epub 2009 Nov 16. Proc Natl Acad Sci U S A. 2009. PMID: 19918060 Free PMC article.
-
Early CD4+ T Cell Responses Are Associated with Subsequent CD8+ T Cell Responses to an rAd5-Based Prophylactic Prime-Boost HIV Vaccine Strategy.PLoS One. 2016 Apr 28;11(4):e0152952. doi: 10.1371/journal.pone.0152952. eCollection 2016. PLoS One. 2016. PMID: 27124598 Free PMC article. Clinical Trial.
-
HIV Susceptibility of human antigen-specific CD4 T cells in AIDS pathogenesis and vaccine response.Expert Rev Vaccines. 2016 Jun;15(6):709-17. doi: 10.1586/14760584.2016.1147354. Epub 2016 Feb 17. Expert Rev Vaccines. 2016. PMID: 26814372 Free PMC article. Review.
-
The "STEP-wise" future of adenovirus-based HIV vaccines.Curr Med Chem. 2011;18(26):3981-6. doi: 10.2174/092986711796957211. Curr Med Chem. 2011. PMID: 21824093 Review.
Cited by
-
Sequential Dysfunction and Progressive Depletion of Candida albicans-Specific CD4 T Cell Response in HIV-1 Infection.PLoS Pathog. 2016 Jun 9;12(6):e1005663. doi: 10.1371/journal.ppat.1005663. eCollection 2016 Jun. PLoS Pathog. 2016. PMID: 27280548 Free PMC article.
-
Regulating Innate and Adaptive Immunity for Controlling SIV Infection by 25-Hydroxycholesterol.Front Immunol. 2018 Nov 21;9:2686. doi: 10.3389/fimmu.2018.02686. eCollection 2018. Front Immunol. 2018. PMID: 30524435 Free PMC article.
-
Distinct susceptibility of HIV vaccine vector-induced CD4 T cells to HIV infection.PLoS Pathog. 2018 Feb 23;14(2):e1006888. doi: 10.1371/journal.ppat.1006888. eCollection 2018 Feb. PLoS Pathog. 2018. PMID: 29474461 Free PMC article.
-
Epigenetic Suppression of HIV in Myeloid Cells by the BRD4-Selective Small Molecule Modulator ZL0580.J Virol. 2020 May 18;94(11):e01880-19. doi: 10.1128/JVI.01880-19. Print 2020 May 18. J Virol. 2020. PMID: 32188727 Free PMC article.
-
Sieve analysis of breakthrough HIV-1 sequences in HVTN 505 identifies vaccine pressure targeting the CD4 binding site of Env-gp120.PLoS One. 2017 Nov 17;12(11):e0185959. doi: 10.1371/journal.pone.0185959. eCollection 2017. PLoS One. 2017. PMID: 29149197 Free PMC article.
References
-
- Shiver JW, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415(6869):331–335. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

