Exocytosis and Endocytosis of Small Vesicles across the Plasma Membrane in Saccharomyces cerevisiae
- PMID: 25192542
- PMCID: PMC4194051
- DOI: 10.3390/membranes4030608
Exocytosis and Endocytosis of Small Vesicles across the Plasma Membrane in Saccharomyces cerevisiae
Abstract
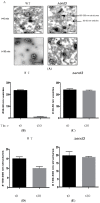
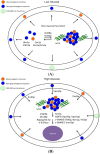
When Saccharomyces cerevisiae is starved of glucose, the gluconeogenic enzymes fructose-1,6-bisphosphatase (FBPase), phosphoenolpyruvate carboxykinase, isocitrate lyase, and malate dehydrogenase, as well as the non-gluconeogenic enzymes glyceraldehyde-3-phosphate dehydrogenase and cyclophilin A, are secreted into the periplasm. In the extracellular fraction, these secreted proteins are associated with small vesicles that account for more than 90% of the total number of extracellular structures observed. When glucose is added to glucose-starved cells, FBPase is internalized and associated with clusters of small vesicles in the cytoplasm. Specifically, the internalization of FBPase results in the decline of FBPase and vesicles in the extracellular fraction and their appearance in the cytoplasm. The clearance of extracellular vesicles and vesicle-associated proteins from the extracellular fraction is dependent on the endocytosis gene END3. This internalization is regulated when cells are transferred from low to high glucose. It is rapidly occurring and is a high capacity process, as clusters of vesicles occupy 10%-20% of the total volume in the cytoplasm in glucose re-fed cells. FBPase internalization also requires the VPS34 gene encoding PI3K. Following internalization, FBPase is delivered to the vacuole for degradation, whereas proteins that are not degraded may be recycled.
Figures




Similar articles
-
The endocytosis gene END3 is essential for the glucose-induced rapid decline of small vesicles in the extracellular fraction in Saccharomyces cerevisiae.J Extracell Vesicles. 2014 Mar 21;3. doi: 10.3402/jev.v3.23497. eCollection 2014. J Extracell Vesicles. 2014. PMID: 24665361 Free PMC article.
-
Vps34p is required for the decline of extracellular fructose-1,6-bisphosphatase in the vacuole import and degradation pathway.J Biol Chem. 2012 Sep 21;287(39):33080-93. doi: 10.1074/jbc.M112.360412. Epub 2012 Jul 25. J Biol Chem. 2012. PMID: 22833678 Free PMC article.
-
Fructose-1,6-bisphosphatase, Malate Dehydrogenase, Isocitrate Lyase, Phosphoenolpyruvate Carboxykinase, Glyceraldehyde-3-phosphate Dehydrogenase, and Cyclophilin A are secreted in Saccharomyces cerevisiae grown in low glucose.Commun Integr Biol. 2013 Nov 1;6(6):e27216. doi: 10.4161/cib.27216. Epub 2013 Dec 10. Commun Integr Biol. 2013. PMID: 24563717 Free PMC article.
-
The key gluconeogenic enzyme fructose-1,6-bisphosphatase is secreted during prolonged glucose starvation and is internalized following glucose re-feeding via the non-classical secretory and internalizing pathways in Saccharomyces cerevisiae.Plant Signal Behav. 2013 Aug;8(8):e24936. doi: 10.4161/psb.24936. Epub 2013 Jun 26. Plant Signal Behav. 2013. PMID: 23673352 Free PMC article. Review.
-
Yeast as a Model System to Study Trafficking of Small Vesicles Carrying Signal-less Proteins In and Out of the Cell.Curr Protein Pept Sci. 2016;17(8):808-820. doi: 10.2174/1389203717666160226144457. Curr Protein Pept Sci. 2016. PMID: 26916166 Review.
Cited by
-
Cell periphery-related proteins as major genomic targets behind the adaptive evolution of an industrial Saccharomyces cerevisiae strain to combined heat and hydrolysate stress.BMC Genomics. 2015 Jul 9;16(1):514. doi: 10.1186/s12864-015-1737-4. BMC Genomics. 2015. PMID: 26156140 Free PMC article.
-
Intracellular vesicle clusters are organelles that synthesize extracellular vesicle-associated cargo proteins in yeast.J Biol Chem. 2020 Feb 28;295(9):2650-2663. doi: 10.1074/jbc.RA119.008612. Epub 2020 Jan 23. J Biol Chem. 2020. PMID: 31974164 Free PMC article.
-
From Tumor Metastasis towards Cerebral Ischemia-Extracellular Vesicles as a General Concept of Intercellular Communication Processes.Int J Mol Sci. 2019 Nov 28;20(23):5995. doi: 10.3390/ijms20235995. Int J Mol Sci. 2019. PMID: 31795140 Free PMC article. Review.
-
Evaluation of Unconventional Protein Secretion by Saccharomyces cerevisiae and other Fungi.Cells. 2018 Aug 31;7(9):128. doi: 10.3390/cells7090128. Cells. 2018. PMID: 30200367 Free PMC article. Review.
-
Extracellular Glycolytic Activities in Root Endophytic Serendipitaceae and Their Regulation by Plant Sugars.Microorganisms. 2022 Jan 29;10(2):320. doi: 10.3390/microorganisms10020320. Microorganisms. 2022. PMID: 35208775 Free PMC article.
References
-
- Carlson M. Regulation of glucose utilization in yeast. Curr. Opin. Genet. Dev. 1998;8:560–564. - PubMed
-
- Fraenkel D.G. The top genes: On the distance from transcript to function in yeast glycolysis. Curr. Opin. Microbiol. 2003;6:198–201. - PubMed
-
- Gancedo J.M. The early steps of glucose signalling in yeast. FEMS Microbiol. Rev. 2008;32:673–704. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

