A study of the expression of small conductance calcium-activated potassium channels (SK1-3) in sensory endings of muscle spindles and lanceolate endings of hair follicles in the rat
- PMID: 25191752
- PMCID: PMC4156425
- DOI: 10.1371/journal.pone.0107073
A study of the expression of small conductance calcium-activated potassium channels (SK1-3) in sensory endings of muscle spindles and lanceolate endings of hair follicles in the rat
Abstract
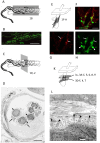
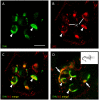
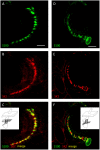
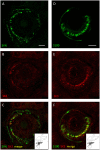
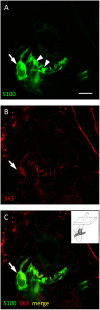
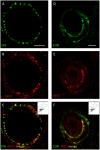
Processes underlying mechanotransduction and its regulation are poorly understood. Inhibitors of Ca2+-activated K+ channels cause a dramatic increase in afferent output from stretched muscle spindles. We used immunocytochemistry to test for the presence and location of small conductance Ca2+-activated K+ channels (SK1-3) in primary endings of muscle spindles and lanceolate endings of hair follicles in the rat. Tissue sections were double immunolabelled with antibodies to one of the SK channel isoforms and to either synaptophysin (SYN, as a marker of synaptic like vesicles (SLV), present in many mechanosensitive endings) or S100 (a Ca2+-binding protein present in glial cells). SK channel immunoreactivity was also compared to immunolabelling for the Na+ ion channel ASIC2, previously reported in both spindle primary and lanceolate endings. SK1 was not detected in sensory terminals of either muscle spindles or lanceolate endings. SK2 was found in the terminals of both muscle spindles and lanceolate endings, where it colocalised with the SLV marker SYN (spindles and lanceolates) and the satellite glial cell (SGC) marker S100 (lanceolates). SK3 was not detected in muscle spindles; by contrast it was present in hair follicle endings, expressed predominantly in SGCs but perhaps also in the SGC: terminal interface, as judged by colocalisation statistical analysis of SYN and S100 immunoreactivity. The possibility that all three isoforms might be expressed in pre-terminal axons, especially at heminodes, cannot be ruled out. Differential distribution of SK channels is likely to be important in their function of responding to changes in intracellular [Ca2+] thereby modulating mechanosensory transduction by regulating the excitability of the sensory terminals. In particular, the presence of SK2 throughout the sensory terminals of both kinds of mechanoreceptor indicates an important role for an outward Ca2+-activated K+ current in the formation of the receptor potential in both types of ending.
Conflict of interest statement
Figures



Similar articles
-
Synaptic-like vesicles and candidate transduction channels in mechanosensory terminals.J Anat. 2015 Aug;227(2):194-213. doi: 10.1111/joa.12337. J Anat. 2015. PMID: 26179025 Free PMC article. Review.
-
Glutamatergic modulation of synaptic-like vesicle recycling in mechanosensory lanceolate nerve terminals of mammalian hair follicles.J Physiol. 2013 May 15;591(10):2523-40. doi: 10.1113/jphysiol.2012.243659. Epub 2013 Feb 25. J Physiol. 2013. PMID: 23440964 Free PMC article.
-
Modulating mechanosensory afferent excitability by an atypical mGluR.J Anat. 2015 Aug;227(2):214-20. doi: 10.1111/joa.12319. Epub 2015 Jun 5. J Anat. 2015. PMID: 26053109 Free PMC article. Review.
-
Distribution of TTX-sensitive voltage-gated sodium channels in primary sensory endings of mammalian muscle spindles.J Neurophysiol. 2017 Apr 1;117(4):1690-1701. doi: 10.1152/jn.00889.2016. Epub 2017 Jan 25. J Neurophysiol. 2017. PMID: 28123009 Free PMC article.
-
Autogenic modulation of mechanoreceptor excitability by glutamate release from synaptic-like vesicles: evidence from the rat muscle spindle primary sensory ending.J Physiol. 2005 Jan 15;562(Pt 2):381-94. doi: 10.1113/jphysiol.2004.074799. Epub 2004 Nov 4. J Physiol. 2005. PMID: 15528245 Free PMC article.
Cited by
-
Vesicle-released glutamate is necessary to maintain muscle spindle afferent excitability but not dynamic sensitivity in adult mice.J Physiol. 2021 Jun;599(11):2953-2967. doi: 10.1113/JP281182. Epub 2021 Apr 18. J Physiol. 2021. PMID: 33749829 Free PMC article.
-
Comprehensive Analysis of Circular RNA Expression in ceRNA Networks and Identification of the Effects of hsa_circ_0006867 in Keloid Dermal Fibroblasts.Front Mol Biosci. 2022 Jan 31;9:800122. doi: 10.3389/fmolb.2022.800122. eCollection 2022. Front Mol Biosci. 2022. PMID: 35174214 Free PMC article.
-
Synaptic-like vesicles and candidate transduction channels in mechanosensory terminals.J Anat. 2015 Aug;227(2):194-213. doi: 10.1111/joa.12337. J Anat. 2015. PMID: 26179025 Free PMC article. Review.
-
Optical Monitoring of Living Nerve Terminal Labeling in Hair Follicle Lanceolate Endings of the Ex Vivo Mouse Ear Skin.J Vis Exp. 2016 Apr 5;(110):e53855. doi: 10.3791/53855. J Vis Exp. 2016. PMID: 27077818 Free PMC article.
-
Molecular determinants of mechanosensation in the muscle spindle.Curr Opin Neurobiol. 2022 Jun;74:102542. doi: 10.1016/j.conb.2022.102542. Epub 2022 Apr 14. Curr Opin Neurobiol. 2022. PMID: 35430481 Free PMC article. Review.
References
-
- Berkefeld H, Fakler B, Schulte U (2010) Ca2+-activated K+ channels: from protein complexes to function. Physiol Rev 90: 1437–1459. - PubMed
-
- Mongan LC, Hill MJ, Chen MX, Tate SN, Collins SD, et al. (2005) The distribution of small and intermediate conductance calcium-activated potassium channels in the rat sensory nervous system. Neurosci 131: 161–175. - PubMed
-
- Kruse MN, Poppele RE (1991) Components of the dynamic response of mammalian muscle spindles that originate in the sensory terminals. Exp Brain Res 86: 359–366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

