Development of HIV reservoir targeted long acting nanoformulated antiretroviral therapies
- PMID: 25174930
- PMCID: PMC4281174
- DOI: 10.2174/0929867321666140826114135
Development of HIV reservoir targeted long acting nanoformulated antiretroviral therapies
Abstract
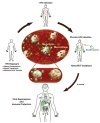
Human immunodeficiency virus (HIV) infection commonly results in a myriad of comorbid conditions secondary to immune deficiency. Infection also affects broad organ system function. Although current antiretroviral therapy (ART) reduces disease morbidity and mortality through effective control of peripheral viral load, restricted infection in HIV reservoirs including gut, lymphoid and central nervous system tissues, is not eliminated. What underlies these events is, in part, poor ART penetrance into each organ across tissue barriers, viral mutation and the longevity of infected cells. We posit that one means to improve these disease outcomes is through nanotechnology. To this end, this review discusses a broad range of cutting-edge nanomedicines and nanomedicine platforms that are or can be used to improve ART delivery. Discussion points include how polymer-drug conjugates, dendrimers, micelles, liposomes, solid lipid nanoparticles and polymeric nanoparticles can be harnessed to best yield cell-based delivery systems. When completely developed, such nanomedicine platforms have the potential to clear reservoirs of viral infection.
Conflict of interest statement
The authors confirm no conflict of interest
Figures


Similar articles
-
Nano-Medicine as a Newly Emerging Approach to Combat Human Immunodeficiency Virus (HIV).Pharm Nanotechnol. 2018;6(1):17-27. doi: 10.2174/2211738506666180209095710. Pharm Nanotechnol. 2018. PMID: 29424324 Review.
-
Progress in antiretroviral drug delivery using nanotechnology.Int J Nanomedicine. 2010 Aug 9;5:533-47. Int J Nanomedicine. 2010. PMID: 20957115 Free PMC article. Review.
-
Long-acting slow effective release antiretroviral therapy.Expert Opin Drug Deliv. 2017 Nov;14(11):1281-1291. doi: 10.1080/17425247.2017.1288212. Epub 2017 Feb 6. Expert Opin Drug Deliv. 2017. PMID: 28128004 Free PMC article. Review.
-
Nanotechnology and the treatment of HIV infection.Viruses. 2012 Apr;4(4):488-520. doi: 10.3390/v4040488. Epub 2012 Apr 10. Viruses. 2012. PMID: 22590683 Free PMC article. Review.
-
Small magnetite antiretroviral therapeutic nanoparticle probes for MRI of drug biodistribution.Nanomedicine (Lond). 2014 Jul;9(9):1341-52. doi: 10.2217/nnm.13.92. Epub 2013 Aug 1. Nanomedicine (Lond). 2014. PMID: 23905578 Free PMC article.
Cited by
-
Antiretroviral Drugs-Loaded Nanoparticles Fabricated by Dispersion Polymerization with Potential for HIV/AIDS Treatment.Infect Dis (Auckl). 2016 Mar 20;9:21-32. doi: 10.4137/IDRT.S38108. eCollection 2016. Infect Dis (Auckl). 2016. PMID: 27013886 Free PMC article.
-
New Therapies and Strategies to Curb HIV Infections with a Focus on Macrophages and Reservoirs.Viruses. 2024 Sep 18;16(9):1484. doi: 10.3390/v16091484. Viruses. 2024. PMID: 39339960 Free PMC article. Review.
-
Tricaprylin-based drug crystalline suspension for intramuscular long-acting delivery of entecavir with alleviated local inflammation.Bioeng Transl Med. 2024 Jan 29;9(4):e10649. doi: 10.1002/btm2.10649. eCollection 2024 Jul. Bioeng Transl Med. 2024. PMID: 39036080 Free PMC article.
-
A review of existing strategies for designing long-acting parenteral formulations: Focus on underlying mechanisms, and future perspectives.Acta Pharm Sin B. 2021 Aug;11(8):2396-2415. doi: 10.1016/j.apsb.2021.05.002. Epub 2021 Jun 17. Acta Pharm Sin B. 2021. PMID: 34522592 Free PMC article. Review.
-
Development of NanoART for HIV Treatment: Minding the Cytochrome P450 (CYP) Enzymes.J Pers Nanomed. 2015;1(1):24-32. Epub 2015 Sep 10. J Pers Nanomed. 2015. PMID: 26635972 Free PMC article.
References
-
- Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature (London) 1996;381(6584):661–666. - PubMed
-
- Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science (Washington, D C) 1996;272(5263):872–877. - PubMed
-
- Zapp ML, Green MR. Sequence-specific RNA binding by the HIV-1 Rev protein. Nature (London) 1989;342(6250):714–716. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS036126/NS/NINDS NIH HHS/United States
- P01 NS043985/NS/NINDS NIH HHS/United States
- 1P01 DA028555/DA/NIDA NIH HHS/United States
- P01MH64570/MH/NIMH NIH HHS/United States
- P01 DA028555/DA/NIDA NIH HHS/United States
- P30 MH062261/MH/NIMH NIH HHS/United States
- P01 NS031492/NS/NINDS NIH HHS/United States
- P01 NS31492/NS/NINDS NIH HHS/United States
- 2R01 NS034239/NS/NINDS NIH HHS/United States
- P20RR 15635/RR/NCRR NIH HHS/United States
- P01 MH064570/MH/NIMH NIH HHS/United States
- R01 NS034239/NS/NINDS NIH HHS/United States
- P01 NS43985/NS/NINDS NIH HHS/United States
- R37 NS036126/NS/NINDS NIH HHS/United States
- R01 AG043540/AG/NIA NIH HHS/United States
- P20 RR015635/RR/NCRR NIH HHS/United States
- 2R37 NS36126/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
