Sustained inflammation and differential expression of interferons type I and III in PVM-infected interferon-gamma (IFNγ) gene-deleted mice
- PMID: 25173090
- PMCID: PMC4253980
- DOI: 10.1016/j.virol.2014.07.039
Sustained inflammation and differential expression of interferons type I and III in PVM-infected interferon-gamma (IFNγ) gene-deleted mice
Abstract
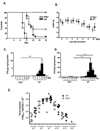
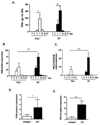
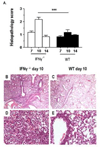
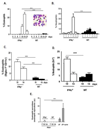
Interferon gamma (IFNγ) has complex immunomodulatory and antiviral properties. While IFNγ is detected in the airways in response to infection with the pneumovirus pathogen, pneumonia virus of mice (PVM; Family Paramyxoviridae), its role in promoting disease has not been fully explored. Here, we evaluate PVM infection in IFNγ(-/-) mice. Although the IFNγ gene-deletion has no impact on weight loss, survival or virus kinetics, expression of IFNβ, IFNλ2/3 and IFN-stimulated 2-5' oligoadenylate synthetases was significantly diminished compared to wild-type counterparts. Furthermore, PVM infection in IFNγ(-/-) mice promoted prominent inflammation, including eosinophil and neutrophil infiltration into the airways and lung parenchyma, observed several days after peak virus titer. Potential mechanisms include over-production of chemoattractant and eosinophil-active cytokines (CXCL1, CCL11, CCL3 and IL5) in PVM-infected IFNγ(-/-) mice; likewise, IFNγ actively antagonized IL5-dependent eosinophil survival ex vivo. Our results may have clinical implications for pneumovirus infection in individuals with IFNγ signaling defects.
Keywords: Eosinophils; Inflammation; Interferon; Pneumovirus.
Published by Elsevier Inc.
Figures


Similar articles
-
Interferon-gamma coordinates CCL3-mediated neutrophil recruitment in vivo.BMC Immunol. 2009 Mar 19;10:14. doi: 10.1186/1471-2172-10-14. BMC Immunol. 2009. PMID: 19298652 Free PMC article.
-
Inflammatory responses to pneumovirus infection in IFN-alpha beta R gene-deleted mice.J Immunol. 2005 Oct 1;175(7):4735-44. doi: 10.4049/jimmunol.175.7.4735. J Immunol. 2005. PMID: 16177121
-
Pulmonary eosinophils and their role in immunopathologic responses to formalin-inactivated pneumonia virus of mice.J Immunol. 2009 Jul 1;183(1):604-12. doi: 10.4049/jimmunol.0802270. J Immunol. 2009. PMID: 19542471 Free PMC article.
-
The Pneumonia Virus of Mice (PVM) model of acute respiratory infection.Viruses. 2012 Dec;4(12):3494-510. doi: 10.3390/v4123494. Viruses. 2012. PMID: 23342367 Free PMC article. Review.
-
Pneumonia virus of mice: severe respiratory infection in a natural host.Immunol Lett. 2008 Jun 15;118(1):6-12. doi: 10.1016/j.imlet.2008.03.013. Epub 2008 Apr 22. Immunol Lett. 2008. PMID: 18471897 Free PMC article. Review.
Cited by
-
Loss of neutrophil polarization in colon carcinoma liver metastases of mice with an inducible, liver-specific IGF-I deficiency.Oncotarget. 2018 Feb 28;9(21):15691-15704. doi: 10.18632/oncotarget.24593. eCollection 2018 Mar 20. Oncotarget. 2018. PMID: 29644002 Free PMC article.
-
Bacterial-induced or passively administered interferon gamma conditions the lung for early control of SARS-CoV-2.Nat Commun. 2023 Dec 12;14(1):8229. doi: 10.1038/s41467-023-43447-0. Nat Commun. 2023. PMID: 38086794 Free PMC article.
-
Interferon gamma modulation of disease manifestation and the local antibody response to alphavirus encephalomyelitis.J Gen Virol. 2016 Nov;97(11):2908-2925. doi: 10.1099/jgv.0.000613. Epub 2016 Sep 22. J Gen Virol. 2016. PMID: 27667782 Free PMC article.
-
Coinfection with Blood-Stage Plasmodium Promotes Systemic Type I Interferon Production during Pneumovirus Infection but Impairs Inflammation and Viral Control in the Lung.Clin Vaccine Immunol. 2015 May;22(5):477-83. doi: 10.1128/CVI.00051-15. Epub 2015 Feb 25. Clin Vaccine Immunol. 2015. PMID: 25716232 Free PMC article.
-
A novel colchicine-myricetin heterozygous molecule: design, synthesis, and effective evaluations on the pathological models of acute lung injury in vitro and in vivo.Front Pharmacol. 2023 Jun 29;14:1224906. doi: 10.3389/fphar.2023.1224906. eCollection 2023. Front Pharmacol. 2023. PMID: 37456754 Free PMC article.
References
-
- Akdis M, Burgler S, Crameri R, Elwegger T, Fujita H, Gomez E, Klunker S, Meyer N, O’Mahony L, Palomares O, Rhyner C, Ouaked N, Schaffartzik A, Van De Veen W, Zeller S, Zimmermann M, Akdis CA. Interleukins, from 1 to 37, and interferon-gamma: receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 2011;127:701–721. - PubMed
-
- Aliberti JCS, Souto JT, Marino APMP, Lannes-Vieira J, Teixeira MM, Farber J, Gazzinelli RT, Silva JS. Modulation of chemokine production and inflammatory responses in interferon-gamma and tumor necrosis factor R1 deficient mice during Trypanosoma cruzi infection. Am. J. Pathol. 2001;158:1433–1440. - PMC - PubMed
-
- Anh DB, Faisca P, Desmecht DJ. Differential resistance / susceptibility patterns to pneumovirus infection among inbred mouse strains. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;291:L426–L435. - PubMed
-
- Boelen A, Kwakkel J, Barends M, de Rond L, Dormans J, Kimman T. Effect of lack of interleukin-4, interleukin-12, interleukin-18 or the interferon-gamma receptor on virus replication, cytokine response and lung pathology during respiratory syncytial virus infection in mice. J. Med. Virol. 2002;66:552–560. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

