T-cell-mediated cross-strain protective immunity elicited by prime-boost vaccination with a live attenuated influenza vaccine
- PMID: 25172265
- PMCID: PMC4197066
- DOI: 10.1016/j.ijid.2014.05.016
T-cell-mediated cross-strain protective immunity elicited by prime-boost vaccination with a live attenuated influenza vaccine
Abstract
Background: Antigenic drift and shift of influenza viruses require frequent reformulation of influenza vaccines. In addition, seasonal influenza vaccines are often mismatched to the epidemic influenza strains. This stresses the need for a universal influenza vaccine.
Methods: BALB/c mice were vaccinated with the trivalent live attenuated (LAIV; FluMist) or inactivated (TIV; FluZone) influenza vaccines and challenged with PR8 (H1N1), FM/47 (H1N1), or HK/68 (H3N2) influenza virus. Cytokines and antibody responses were tested by ELISA. Furthermore, different LAIV dosages were applied in BALB/c mice. LAIV vaccinated mice were also depleted of T-cells and challenged with PR8 virus.
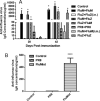
Results: LAIV induced significant protection against challenge with the non-vaccine strain PR8 influenza virus. Furthermore, protective immunity against PR8 was dose-dependent. Of note, interleukin 2 and interferon gamma cytokine secretion in the lung alveolar fluid were significantly elevated in mice vaccinated with LAIV. Moreover, T-cell depletion of LAIV vaccinated mice compromised protection, indicating that T-cell-mediated immunity is required. In contrast, passive transfer of sera from mice vaccinated with LAIV into naïve mice failed to protect against PR8 challenge. Neutralization assays in vitro confirmed that LAIV did not induce cross-strain neutralizing antibodies against PR8 virus. Finally, we showed that three doses of LAIV also provided protection against challenge with two additional heterologous viruses, FM/47 and HK/68.
Conclusions: These results support the potential use of the LAIV as a universal influenza vaccine under a prime-boost vaccination regimen.
Keywords: Cross-strain protective immunity; Heterologous influenza viruses; Live attenuated influenza vaccine; Prime–boost vaccination; T-cell-mediated immunity; Trivalent inactivated influenza vaccine.
Copyright © 2014 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
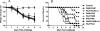
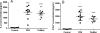
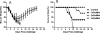
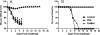
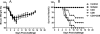
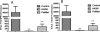
Similar articles
-
Sequential Immunization With Live-Attenuated Chimeric Hemagglutinin-Based Vaccines Confers Heterosubtypic Immunity Against Influenza A Viruses in a Preclinical Ferret Model.Front Immunol. 2019 Apr 10;10:756. doi: 10.3389/fimmu.2019.00756. eCollection 2019. Front Immunol. 2019. PMID: 31105689 Free PMC article.
-
Rationale for vaccination with trivalent or quadrivalent live attenuated influenza vaccines: Protective vaccine efficacy in the ferret model.PLoS One. 2018 Dec 3;13(12):e0208028. doi: 10.1371/journal.pone.0208028. eCollection 2018. PLoS One. 2018. PMID: 30507951 Free PMC article.
-
A Live Attenuated Influenza Vaccine Elicits Enhanced Heterologous Protection When the Internal Genes of the Vaccine Are Matched to Those of the Challenge Virus.J Virol. 2020 Jan 31;94(4):e01065-19. doi: 10.1128/JVI.01065-19. Print 2020 Jan 31. J Virol. 2020. PMID: 31748399 Free PMC article.
-
Novel Approaches for The Development of Live Attenuated Influenza Vaccines.Viruses. 2019 Feb 22;11(2):190. doi: 10.3390/v11020190. Viruses. 2019. PMID: 30813325 Free PMC article. Review.
-
Immune responses after live attenuated influenza vaccination.Hum Vaccin Immunother. 2018 Mar 4;14(3):571-578. doi: 10.1080/21645515.2017.1377376. Epub 2018 Jan 3. Hum Vaccin Immunother. 2018. PMID: 28933664 Free PMC article. Review.
Cited by
-
Immune Responses Elicited by Live Attenuated Influenza Vaccines as Correlates of Universal Protection against Influenza Viruses.Vaccines (Basel). 2021 Apr 7;9(4):353. doi: 10.3390/vaccines9040353. Vaccines (Basel). 2021. PMID: 33916924 Free PMC article. Review.
-
Pneumococcal colonization impairs mucosal immune responses to live attenuated influenza vaccine.JCI Insight. 2021 Feb 22;6(4):e141088. doi: 10.1172/jci.insight.141088. JCI Insight. 2021. PMID: 33497364 Free PMC article. Clinical Trial.
-
Efficacy of an Adenoviral Vectored Multivalent Centralized Influenza Vaccine.Sci Rep. 2017 Nov 2;7(1):14912. doi: 10.1038/s41598-017-14891-y. Sci Rep. 2017. PMID: 29097763 Free PMC article.
-
Harnessing the Power of T Cells: The Promising Hope for a Universal Influenza Vaccine.Vaccines (Basel). 2018 Mar 26;6(2):18. doi: 10.3390/vaccines6020018. Vaccines (Basel). 2018. PMID: 29587436 Free PMC article. Review.
-
Impact of Pre-Existing Immunity on Live Attenuated Influenza Vaccine-Induced Cross-Protective Immunity.Vaccines (Basel). 2020 Aug 20;8(3):459. doi: 10.3390/vaccines8030459. Vaccines (Basel). 2020. PMID: 32825218 Free PMC article.
References
-
- Horimoto T, Kawaoka Y. Influenza: lessons from past pandemics, warnings from current incidents. Nat Rev Microbiol. 2005;3:591–600. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

