The complexity of the calretinin-expressing progenitors in the human cerebral cortex
- PMID: 25165435
- PMCID: PMC4131197
- DOI: 10.3389/fnana.2014.00082
The complexity of the calretinin-expressing progenitors in the human cerebral cortex
Abstract
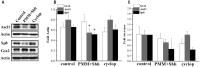
The complex structure and function of the cerebral cortex critically depend on the balance of excitation and inhibition provided by the pyramidal projection neurons and GABAergic interneurons, respectively. The calretinin-expressing (CalR(+)) cell is a subtype of GABAergic cortical interneurons that is more prevalent in humans than in rodents. In rodents, CalR(+) interneurons originate in the caudal ganglionic eminence (CGE) from Gsx2(+) progenitors, but in humans it has been suggested that a subpopulation of CalR(+) cells can also be generated in the cortical ventricular/subventricular zone (VZ/SVZ). The progenitors for cortically generated CalR(+) subpopulation in primates are not yet characterized. Hence, the aim of this study was to identify patterns of expression of the transcription factors (TFs) that commit cortical stem cells to the CalR fate, with a focus on Gsx2. First, we studied the expression of Gsx2 and its downstream effectors, Ascl1 and Sp8 in the cortical regions of the fetal human forebrain at midgestation. Next, we established that a subpopulation of cells expressing these TFs are proliferating in the cortical SVZ, and can be co-labeled with CalR. The presence and proliferation of Gsx2(+) cells, not only in the ventral telencephalon (GE) as previously reported, but also in the cerebral cortex suggests cortical origin of a subpopulation of CalR(+) neurons in humans. In vitro treatment of human cortical progenitors with Sonic hedgehog (Shh), an important morphogen in the specification of interneurons, decreased levels of Ascl1 and Sp8 proteins, but did not affect Gsx2 levels. Taken together, our ex-vivo and in vitro results on human fetal brain suggest complex endogenous and exogenous regulation of TFs implied in the specification of different subtypes of CalR(+) cortical interneurons.
Keywords: GABA; Gsx2; cortical development; cortical interneurons; transcription factors.
Figures
Similar articles
-
Dorsal radial glial cells have the potential to generate cortical interneurons in human but not in mouse brain.J Neurosci. 2011 Feb 16;31(7):2413-20. doi: 10.1523/JNEUROSCI.5249-10.2011. J Neurosci. 2011. PMID: 21325508 Free PMC article.
-
Spatio-temporal extension in site of origin for cortical calretinin neurons in primates.Front Neuroanat. 2014 Jun 26;8:50. doi: 10.3389/fnana.2014.00050. eCollection 2014. Front Neuroanat. 2014. PMID: 25018702 Free PMC article. Review.
-
Characterization of Glcci1 expression in a subpopulation of lateral ganglionic eminence progenitors in the mouse telencephalon.Dev Dyn. 2018 Jan;247(1):222-228. doi: 10.1002/dvdy.24556. Epub 2017 Aug 18. Dev Dyn. 2018. PMID: 28744915 Free PMC article.
-
Expression of ventral telencephalon transcription factors ASCL1 and DLX2 in the early fetal human cerebral cortex.J Anat. 2019 Sep;235(3):555-568. doi: 10.1111/joa.12971. Epub 2019 Mar 12. J Anat. 2019. PMID: 30861584 Free PMC article.
-
Revisiting enigmatic cortical calretinin-expressing interneurons.Front Neuroanat. 2014 Jun 24;8:52. doi: 10.3389/fnana.2014.00052. eCollection 2014. Front Neuroanat. 2014. PMID: 25009470 Free PMC article. Review.
Cited by
-
Estrogen Treatment Reverses Prematurity-Induced Disruption in Cortical Interneuron Population.J Neurosci. 2018 Aug 22;38(34):7378-7391. doi: 10.1523/JNEUROSCI.0478-18.2018. Epub 2018 Jul 23. J Neurosci. 2018. PMID: 30037831 Free PMC article.
-
Secretagogin is Expressed by Developing Neocortical GABAergic Neurons in Humans but not Mice and Increases Neurite Arbor Size and Complexity.Cereb Cortex. 2018 Jun 1;28(6):1946-1958. doi: 10.1093/cercor/bhx101. Cereb Cortex. 2018. PMID: 28449024 Free PMC article.
-
Comparative features of calretinin, calbindin, and parvalbumin expressing interneurons in mouse and monkey primary visual and frontal cortices.J Comp Neurol. 2023 Dec;531(18):1934-1962. doi: 10.1002/cne.25514. Epub 2023 Jun 26. J Comp Neurol. 2023. PMID: 37357562 Free PMC article.
-
Comparative Features of Calretinin, Calbindin and Parvalbumin Expressing Interneurons in Mouse and Monkey Primary Visual and Frontal Cortices.bioRxiv [Preprint]. 2023 Feb 28:2023.02.27.530269. doi: 10.1101/2023.02.27.530269. bioRxiv. 2023. Update in: J Comp Neurol. 2023 Dec;531(18):1934-1962. doi: 10.1002/cne.25514. PMID: 36909556 Free PMC article. Updated. Preprint.
-
Prenatal alcohol exposure is a leading cause of interneuronopathy in humans.Acta Neuropathol Commun. 2020 Nov 30;8(1):208. doi: 10.1186/s40478-020-01089-z. Acta Neuropathol Commun. 2020. PMID: 33256853 Free PMC article.
References
-
- Bayatti N., Moss J. A., Sun L., Ambrose P., Ward J. F. H., Lindsay S., et al. (2008). A molecular neuroanatomical study of the developing human neocortex from 8 to 17 postconceptional weeks revealing the early differentiation of the subplate and subventricular xone. Cereb. Cortex 18 1536–1548 10.1093/cercor/bhm184 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

