A model of the medial superior olive explains spatiotemporal features of local field potentials
- PMID: 25164666
- PMCID: PMC4145174
- DOI: 10.1523/JNEUROSCI.0175-14.2014
A model of the medial superior olive explains spatiotemporal features of local field potentials
Abstract
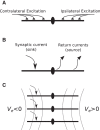
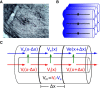
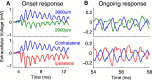
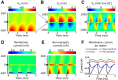
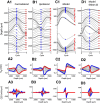
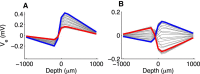
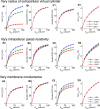
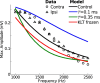
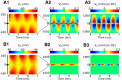
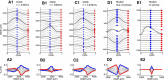
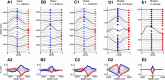
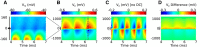
Local field potentials are important indicators of in vivo neural activity. Sustained, phase-locked, sound-evoked extracellular fields in the mammalian auditory brainstem, known as the auditory neurophonic, reflect the activity of neurons in the medial superior olive (MSO). We develop a biophysically based model of the neurophonic that accounts for features of in vivo extracellular recordings in the cat auditory brainstem. By making plausible idealizations regarding the spatial symmetry of MSO neurons and the temporal synchrony of their afferent inputs, we reduce the challenging problem of computing extracellular potentials in a 3D volume conductor to a one-dimensional problem. We find that postsynaptic currents in bipolar MSO neuron models generate extracellular voltage responses that strikingly resemble in vivo recordings. Simulations reproduce distinctive spatiotemporal features of the in vivo neurophonic response to monaural pure tones: large oscillations (hundreds of microvolts to millivolts), broad spatial reach (millimeter scale), and a dipole-like spatial profile. We also explain how somatic inhibition and the relative timing of bilateral excitation may shape the spatial profile of the neurophonic. We observe in simulations, and find supporting evidence in in vivo data, that coincident excitatory inputs on both dendrites lead to a drastically reduced spatial reach of the neurophonic. This outcome surprises because coincident inputs are thought to evoke maximal firing rates in MSO neurons, and it reconciles previously puzzling evoked potential results in humans and animals. The success of our model, which has no axon or spike-generating sodium currents, suggests that MSO spikes do not contribute appreciably to the neurophonic.
Keywords: auditory brainstem; computer model; local field potential; medial superior olive; neurophonic.
Copyright © 2014 the authors 0270-6474/14/3411705-18$15.00/0.
Figures
Similar articles
-
Signatures of Somatic Inhibition and Dendritic Excitation in Auditory Brainstem Field Potentials.J Neurosci. 2017 Oct 25;37(43):10451-10467. doi: 10.1523/JNEUROSCI.0600-17.2017. Epub 2017 Sep 25. J Neurosci. 2017. PMID: 28947575 Free PMC article.
-
Oscillatory dipoles as a source of phase shifts in field potentials in the mammalian auditory brainstem.J Neurosci. 2010 Oct 6;30(40):13472-87. doi: 10.1523/JNEUROSCI.0294-10.2010. J Neurosci. 2010. PMID: 20926673 Free PMC article.
-
Somatic Integration of Incoherent Dendritic Inputs in the Gerbil Medial Superior Olive.J Neurosci. 2023 May 31;43(22):4093-4109. doi: 10.1523/JNEUROSCI.2215-22.2023. Epub 2023 May 2. J Neurosci. 2023. PMID: 37130779 Free PMC article.
-
Synaptic integration in dendrites: exceptional need for speed.J Physiol. 2012 Nov 15;590(22):5563-9. doi: 10.1113/jphysiol.2012.229328. Epub 2012 Aug 28. J Physiol. 2012. PMID: 22930273 Free PMC article. Review.
-
The evolution of temporal processing in the medial superior olive, an auditory brainstem structure.Prog Neurobiol. 2000 Aug;61(6):581-610. doi: 10.1016/s0301-0082(99)00068-4. Prog Neurobiol. 2000. PMID: 10775798 Review.
Cited by
-
Maps of interaural delay in the owl's nucleus laminaris.J Neurophysiol. 2015 Sep;114(3):1862-73. doi: 10.1152/jn.00644.2015. Epub 2015 Jul 29. J Neurophysiol. 2015. PMID: 26224776 Free PMC article.
-
The Calyx of Held: A Hypothesis on the Need for Reliable Timing in an Intensity-Difference Encoder.Neuron. 2018 Nov 7;100(3):534-549. doi: 10.1016/j.neuron.2018.10.026. Neuron. 2018. PMID: 30408442 Free PMC article. Review.
-
Signatures of Somatic Inhibition and Dendritic Excitation in Auditory Brainstem Field Potentials.J Neurosci. 2017 Oct 25;37(43):10451-10467. doi: 10.1523/JNEUROSCI.0600-17.2017. Epub 2017 Sep 25. J Neurosci. 2017. PMID: 28947575 Free PMC article.
-
The Binaural Interaction Component in Barn Owl (Tyto alba) Presents few Differences to Mammalian Data.J Assoc Res Otolaryngol. 2016 Dec;17(6):577-589. doi: 10.1007/s10162-016-0583-7. Epub 2016 Aug 25. J Assoc Res Otolaryngol. 2016. PMID: 27562803 Free PMC article.
-
Neuronal coupling by endogenous electric fields: cable theory and applications to coincidence detector neurons in the auditory brain stem.J Neurophysiol. 2016 Apr;115(4):2033-51. doi: 10.1152/jn.00780.2015. Epub 2016 Jan 28. J Neurophysiol. 2016. PMID: 26823512 Free PMC article.
References
-
- Biedenbach MA, Freeman WJ. Click-evoked potential map from the superior olivary nucleus. Am J Physiol. 1964;206:1408–1414. - PubMed
-
- Blackburn CC, Sachs MB. Classification of unit types in the anteroventral cochlear nucleus: PST histograms and regularity analysis. J Neurophysiol. 1989;62:1303–1329. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
