Retinoblastoma binding protein 2 (RBP2) promotes HIF-1α-VEGF-induced angiogenesis of non-small cell lung cancer via the Akt pathway
- PMID: 25162518
- PMCID: PMC4146555
- DOI: 10.1371/journal.pone.0106032
Retinoblastoma binding protein 2 (RBP2) promotes HIF-1α-VEGF-induced angiogenesis of non-small cell lung cancer via the Akt pathway
Abstract
Background: Pathological angiogenesis plays an essential role in tumor aggressiveness and leads to unfavorable prognosis. The aim of this study is to detect the potential role of Retinoblastoma binding protein 2 (RBP2) in the tumor angiogenesis of non-small cell lung cancer (NSCLC).
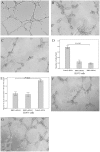
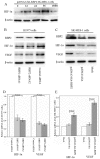
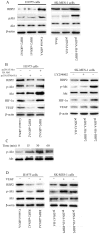
Methods: Immunohistochemical staining was used to detect the expression of RBP2, hypoxia-inducible factor-1α (HIF-1α), vascular endothelial growth factor (VEGF) and CD34. Two pairs of siRNA sequences and pcDNA3-HA-RBP2 were used to down-regulate and up-regulate RBP2 expression in H1975 and SK-MES-1 cells. An endothelial cell tube formation assay, VEGF enzyme-linked immunosorbent assay, real-time PCR and western blotting were performed to detect the potential mechanisms mediated by RBP2 in tumor angiogenesis.
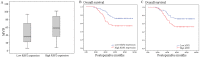
Results: Of the 102 stage I NSCLC specimens analyzed, high RBP2 protein expression is closely associated with tumor size (P = 0.030), high HIF-1α expression (P = 0.028), high VEGF expression (P = 0.048), increased tumor angiogenesis (P = 0.033) and poor prognosis (P = 0.037); high MVD was associated with high HIF-1α expression (P = 0.034), high VEGF expression (P = 0.001) and poor prognosis (P = 0.040). Multivariate analysis indicated that RBP2 had an independent influence on the survival of patients with stage I NSCLC (P = 0.044). By modulating the expression of RBP2, our findings suggested that RBP2 protein depletion decreased HUVECs tube formation by down-regulating VEGF in a conditioned medium. RBP2 stimulated the up-regulation of VEGF, which was dependent on HIF-1α, and activated the HIF-1α via phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway. Moreover, VEGF increased the activation of Akt regulated by RBP2.
Conclusions: The RBP2 protein may stimulate HIF-1α expression via the activation of the PI3K/Akt signaling pathway under normoxia and then stimulate VEGF expression. These findings indicate that RBP2 may play a critical role in tumor angiogenesis and serve as an attractive therapeutic target against tumor aggressiveness for early-stage NSCLC patients.
Conflict of interest statement
Figures



Similar articles
-
Roles of PI3K/Akt and c-Jun signaling pathways in human papillomavirus type 16 oncoprotein-induced HIF-1α, VEGF, and IL-8 expression and in vitro angiogenesis in non-small cell lung cancer cells.PLoS One. 2014 Jul 24;9(7):e103440. doi: 10.1371/journal.pone.0103440. eCollection 2014. PLoS One. 2014. PMID: 25058399 Free PMC article.
-
Influence of vascular endothelial growth factor single nucleotide polymorphisms on non-small cell lung cancer tumor angiogenesis.Oncol Rep. 2013 Jan;29(1):39-44. doi: 10.3892/or.2012.2075. Epub 2012 Oct 9. Oncol Rep. 2013. PMID: 23064377 Free PMC article.
-
(-)-Epigallocatechin-3-gallate inhibits human papillomavirus (HPV)-16 oncoprotein-induced angiogenesis in non-small cell lung cancer cells by targeting HIF-1α.Cancer Chemother Pharmacol. 2013 Mar;71(3):713-25. doi: 10.1007/s00280-012-2063-z. Epub 2013 Jan 6. Cancer Chemother Pharmacol. 2013. PMID: 23292117
-
Clinicopathological and prognostic significance of hypoxia-inducible factor-1 alpha in lung cancer: a systematic review with meta-analysis.J Huazhong Univ Sci Technolog Med Sci. 2016 Jun;36(3):321-327. doi: 10.1007/s11596-016-1586-7. Epub 2016 Jul 5. J Huazhong Univ Sci Technolog Med Sci. 2016. PMID: 27376798 Review.
-
Dual Roles of the AMP-Activated Protein Kinase Pathway in Angiogenesis.Cells. 2019 Jul 19;8(7):752. doi: 10.3390/cells8070752. Cells. 2019. PMID: 31331111 Free PMC article. Review.
Cited by
-
A prognostic model of non small cell lung cancer based on TCGA and ImmPort databases.Sci Rep. 2022 Jan 10;12(1):437. doi: 10.1038/s41598-021-04268-7. Sci Rep. 2022. PMID: 35013450 Free PMC article.
-
Unraveling the Proteomic Landscape of Intestinal Epithelial Cell-Derived Exosomes in Mice.Front Physiol. 2022 Feb 23;13:773671. doi: 10.3389/fphys.2022.773671. eCollection 2022. Front Physiol. 2022. PMID: 35283765 Free PMC article.
-
Sub-lethal Doses of Polybrominated Diphenyl Ethers, in Vitro, Promote Oxidative Stress and Modulate Molecular Markers Related to Cell Cycle, Antioxidant Balance and Cellular Energy Management.Int J Environ Res Public Health. 2019 Feb 18;16(4):588. doi: 10.3390/ijerph16040588. Int J Environ Res Public Health. 2019. PMID: 30781636 Free PMC article.
-
MicroRNA‑363‑3p inhibits cell proliferation and induces apoptosis in retinoblastoma cells via the Akt/mTOR signaling pathway by targeting PIK3CA.Oncol Rep. 2020 May;43(5):1365-1374. doi: 10.3892/or.2020.7544. Epub 2020 Mar 12. Oncol Rep. 2020. PMID: 32323827 Free PMC article.
-
Decorin is responsible for progression of non-small-cell lung cancer by promoting cell proliferation and metastasis.Tumour Biol. 2015 May;36(5):3345-54. doi: 10.1007/s13277-014-2968-8. Epub 2014 Dec 20. Tumour Biol. 2015. PMID: 25524578
References
-
- Jemal A, Bray F (2011) Center MM, Ferlay J, Ward E, et al (2011) Global cancer statistics. CA Cancer J Clin 61: 69–90. - PubMed
-
- Pisters KM, Evans WK, Azzoli CG, Kris MG, Smith CA, et al. (2007) Cancer Care Ontario and American Society of Clinical Oncology adjuvant chemotherapy and adjuvant radiation therapy for stages I-IIIA resectable non small-cell lung cancer guideline. J Clin Oncol 25: 5506–5518. - PubMed
-
- Vamesu S (2008) Angiogenesis and tumor histologic type in primary breast cancer patients: an analysis of 155 needle core biopsies. Rom J Morphol Embryol 49: 181–188. - PubMed
-
- Vermeulen PB, van Golen KL, Dirix LY (2010) Angiogenesis, lymphangiogenesis, growth pattern, and tumor emboli in inflammatory breast cancer: a review of the current knowledge. Cancer 116: 2748–2754. - PubMed
-
- Tammela T, Enholm B, Alitalo K, Paavonen K (2005) The biology of vascular endothelial growth factors. Cardiovasc Res 65: 550–563. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

