Isolation and characterization of platelet-derived extracellular vesicles
- PMID: 25147646
- PMCID: PMC4125723
- DOI: 10.3402/jev.v3.24692
Isolation and characterization of platelet-derived extracellular vesicles
Abstract
Background: Platelet-derived extracellular vesicles (EVs) participate, for example, in haemostasis, immunity and development. Most studies of platelet EVs have targeted microparticles, whereas exosomes and EV characterization under various conditions have been less analyzed. Studies have been hampered by the difficulty in obtaining EVs free from contaminating cells and platelet remnants. Therefore, we optimized an EV isolation protocol and compared the quantity and protein content of EVs induced by different agonists.
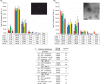
Methods: Platelets isolated with iodixanol gradient were activated by thrombin and collagen, lipopolysaccharide (LPS) or Ca(2+) ionophore. Microparticles and exosomes were isolated by differential centrifugations. EVs were quantitated by nanoparticle tracking analysis (NTA) and total protein. Size distributions were determined by NTA and electron microscopy. Proteomics was used to characterize the differentially induced EVs.
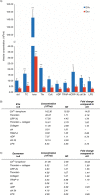
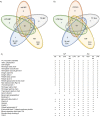
Results: The main EV populations were 100-250 nm and over 90% were <500 nm irrespective of the activation. However, activation pathways differentially regulated the quantity and the quality of EVs, which also formed constitutively. Thrombogenic activation was the most potent physiological EV-generator. LPS was a weak inducer of EVs, which had a selective protein content from the thrombogenic EVs. Ca(2+) ionophore generated a large population of protein-poor and unselectively packed EVs. By proteomic analysis, EVs were highly heterogeneous after the different activations and between the vesicle subpopulations.
Conclusions: Although platelets constitutively release EVs, vesiculation can be increased, and the activation pathway determines the number and the cargo of the formed EVs. These activation-dependent variations render the use of protein content in sample normalization invalid. Since most platelet EVs are 100-250 nm, only a fraction has been analyzed by previously used methods, for example, flow cytometry. As the EV subpopulations could not be distinguished and large vesicle populations may be lost by differential centrifugation, novel methods are required for the isolation and the differentiation of all EVs.
Keywords: exosome; extracellular vesicles; microparticle; microvesicle; nanoparticle tracking analysis; platelet; proteomics; transmission electron microscopy.
Figures



Similar articles
-
Comparing small urinary extracellular vesicle purification methods with a view to RNA sequencing-Enabling robust and non-invasive biomarker research.Biomol Detect Quantif. 2019 Jun 4;17:100089. doi: 10.1016/j.bdq.2019.100089. eCollection 2019 Mar. Biomol Detect Quantif. 2019. PMID: 31194192 Free PMC article.
-
Lipidomic and proteomic characterization of platelet extracellular vesicle subfractions from senescent platelets.Transfusion. 2015 Mar;55(3):507-21. doi: 10.1111/trf.12874. Epub 2014 Oct 21. Transfusion. 2015. PMID: 25332113
-
Extracellular vesicles from activated platelets: a semiquantitative cryo-electron microscopy and immuno-gold labeling study.Platelets. 2017 May;28(3):263-271. doi: 10.1080/09537104.2016.1268255. Epub 2017 Jan 19. Platelets. 2017. PMID: 28102751 Review.
-
Proteomics profiling identifies extracellular vesicles' cargo associated with tumour cell induced platelet aggregation.BMC Cancer. 2022 Sep 29;22(1):1023. doi: 10.1186/s12885-022-10068-7. BMC Cancer. 2022. PMID: 36171564 Free PMC article.
-
Comparison of serum and plasma as a source of blood extracellular vesicles: Increased levels of platelet-derived particles in serum extracellular vesicle fractions alter content profiles from plasma extracellular vesicle fractions.PLoS One. 2022 Jun 24;17(6):e0270634. doi: 10.1371/journal.pone.0270634. eCollection 2022. PLoS One. 2022. PMID: 35749554 Free PMC article. Review.
Cited by
-
Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes.Semin Cell Dev Biol. 2015 Apr;40:41-51. doi: 10.1016/j.semcdb.2015.02.010. Epub 2015 Feb 23. Semin Cell Dev Biol. 2015. PMID: 25721812 Free PMC article. Review.
-
The value of platelet-rich plasma-derived extracellular vesicles in modern medicine.Ann Med. 2023 Dec;55(2):2287705. doi: 10.1080/07853890.2023.2287705. Epub 2023 Dec 8. Ann Med. 2023. PMID: 38065677 Free PMC article. Review.
-
The effect of ionising radiation on the phenotype of bone marrow-derived extracellular vesicles.Br J Radiol. 2020 Nov 1;93(1115):20200319. doi: 10.1259/bjr.20200319. Epub 2020 Sep 30. Br J Radiol. 2020. PMID: 32997527 Free PMC article.
-
Emerging roles of platelet concentrates and platelet-derived extracellular vesicles in regenerative periodontology and implant dentistry.APL Bioeng. 2022 Sep 1;6(3):031503. doi: 10.1063/5.0099872. eCollection 2022 Sep. APL Bioeng. 2022. PMID: 36061076 Free PMC article. Review.
-
Mode of induction of platelet-derived extracellular vesicles is a critical determinant of their phenotype and function.Sci Rep. 2020 Oct 22;10(1):18061. doi: 10.1038/s41598-020-73005-3. Sci Rep. 2020. PMID: 33093473 Free PMC article.
References
-
- Elzey BD, Sprague DL, Ratliff TL. The emerging role of platelets in adaptive immunity. Cell Immunol. 2005;238:1–9. - PubMed
-
- Jenne CN, Urrutia R, Kubes P. Platelets: bridging hemostasis, inflammation, and immunity. Int J Lab Hematol. 2013;35:254–61. - PubMed
-
- Aatonen M, Gronholm M, Siljander PR. Platelet-derived microvesicles: multitalented participants in intercellular communication. Semin Thromb Hemost. 2012;38:102–13. - PubMed
-
- Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94:3791–9. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

