Physicochemical mechanisms of protein regulation by phosphorylation
- PMID: 25147561
- PMCID: PMC4124799
- DOI: 10.3389/fgene.2014.00270
Physicochemical mechanisms of protein regulation by phosphorylation
Abstract
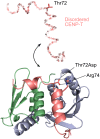
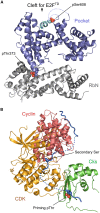
Phosphorylation offers a dynamic way to regulate protein activity and subcellular localization, which is achieved through its reversibility and fast kinetics. Adding or removing a dianionic phosphate group somewhere on a protein often changes the protein's structural properties, its stability and dynamics. Moreover, the majority of signaling pathways involve an extensive set of protein-protein interactions, and phosphorylation can be used to regulate and modulate protein-protein binding. Losses of phosphorylation sites, as a result of disease mutations, might disrupt protein binding and deregulate signal transduction. In this paper we focus on the effects of phosphorylation on protein stability, dynamics, and binding. We describe several physico-chemical mechanisms of protein regulation through phosphorylation and pay particular attention to phosphorylation in protein complexes and phosphorylation in the context of disorder-order and order-disorder transitions. Finally we assess the role of multiple phosphorylation sites in a protein molecule, their possible cooperativity and function.
Keywords: allosteric regulation; multisite phosphorylation; protein disorder; protein phosphorylation; protein–protein interactions.
Figures
Similar articles
-
Regulation of protein-protein binding by coupling between phosphorylation and intrinsic disorder: analysis of human protein complexes.Mol Biosyst. 2013 Jul;9(7):1620-6. doi: 10.1039/c3mb25514j. Epub 2013 Jan 30. Mol Biosyst. 2013. PMID: 23364837 Free PMC article.
-
Versatile regulation of multisite protein phosphorylation by the order of phosphate processing and protein-protein interactions.FEBS J. 2007 Feb;274(4):1046-61. doi: 10.1111/j.1742-4658.2007.05653.x. Epub 2007 Jan 25. FEBS J. 2007. PMID: 17257173
-
Macromolecular crowding: chemistry and physics meet biology (Ascona, Switzerland, 10-14 June 2012).Phys Biol. 2013 Aug;10(4):040301. doi: 10.1088/1478-3975/10/4/040301. Epub 2013 Aug 2. Phys Biol. 2013. PMID: 23912807
-
Targeting protein-protein interactions in complexes organized by A kinase anchoring proteins.Front Pharmacol. 2015 Sep 8;6:192. doi: 10.3389/fphar.2015.00192. eCollection 2015. Front Pharmacol. 2015. PMID: 26441649 Free PMC article. Review.
-
Dynamic cooperativity of molecular processes in active streaming, muscle contraction, and subcellular dynamics: the molecular mechanism of self-organization at the subcellular level.Adv Biophys. 1979;13:195-278. Adv Biophys. 1979. PMID: 161690 Review.
Cited by
-
Monitoring protein phosphorylation by acrylamide pendant Phos-Tag™ in various plants.Front Plant Sci. 2015 May 13;6:336. doi: 10.3389/fpls.2015.00336. eCollection 2015. Front Plant Sci. 2015. PMID: 26029234 Free PMC article.
-
Serine-129 phosphorylation of α-synuclein is an activity-dependent trigger for physiologic protein-protein interactions and synaptic function.Neuron. 2023 Dec 20;111(24):4006-4023.e10. doi: 10.1016/j.neuron.2023.11.020. Neuron. 2023. PMID: 38128479 Free PMC article.
-
Protein Targeting to Glycogen (PTG): A Promising Player in Glucose and Lipid Metabolism.Biomolecules. 2022 Nov 26;12(12):1755. doi: 10.3390/biom12121755. Biomolecules. 2022. PMID: 36551183 Free PMC article. Review.
-
Effect of magnitude and variability of energy of activation in multisite ultrasensitive biochemical processes.PLoS Comput Biol. 2020 Aug 6;16(8):e1007966. doi: 10.1371/journal.pcbi.1007966. eCollection 2020 Aug. PLoS Comput Biol. 2020. PMID: 32760072 Free PMC article.
-
Interaction of the Hippo Pathway and Phosphatases in Tumorigenesis.Cancers (Basel). 2020 Aug 27;12(9):2438. doi: 10.3390/cancers12092438. Cancers (Basel). 2020. PMID: 32867200 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

