Ly6Chi monocyte recruitment is responsible for Th2 associated host-protective macrophage accumulation in liver inflammation due to schistosomiasis
- PMID: 25144366
- PMCID: PMC4140849
- DOI: 10.1371/journal.ppat.1004282
Ly6Chi monocyte recruitment is responsible for Th2 associated host-protective macrophage accumulation in liver inflammation due to schistosomiasis
Abstract
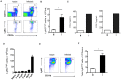
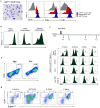
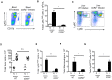
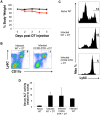
Accumulation of M2 macrophages in the liver, within the context of a strong Th2 response, is a hallmark of infection with the parasitic helminth, Schistosoma mansoni, but the origin of these cells is unclear. To explore this, we examined the relatedness of macrophages to monocytes in this setting. Our data show that both monocyte-derived and resident macrophages are engaged in the response to infection. Infection caused CCR2-dependent increases in numbers of Ly6Chi monocytes in blood and liver and of CX3CR1+ macrophages in diseased liver. Ly6Chi monocytes recovered from liver had the potential to differentiate into macrophages when cultured with M-CSF. Using pulse chase BrdU labeling, we found that most hepatic macrophages in infected mice arose from monocytes. Consistent with this, deletion of monocytes led to the loss of a subpopulation of hepatic CD11chi macrophages that was present in infected but not naïve mice. This was accompanied by a reduction in the size of egg-associated granulomas and significantly exacerbated disease. In addition to the involvement of monocytes and monocyte-derived macrophages in hepatic inflammation due to infection, we observed increased incorporation of BrdU and expression of Ki67 and MHC II in resident macrophages, indicating that these cells are participating in the response. Expression of both M2 and M1 marker genes was increased in liver from infected vs. naive mice. The M2 fingerprint in the liver was not accounted for by a single cell type, but rather reflected expression of M2 genes by various cells including macrophages, neutrophils, eosinophils and monocytes. Our data point to monocyte recruitment as the dominant process for increasing macrophage cell numbers in the liver during schistosomiasis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

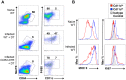
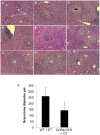
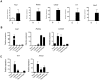
Similar articles
-
Ly6C(high) monocytes become alternatively activated macrophages in schistosome granulomas with help from CD4+ cells.PLoS Pathog. 2014 Jun 26;10(6):e1004080. doi: 10.1371/journal.ppat.1004080. eCollection 2014 Jun. PLoS Pathog. 2014. PMID: 24967715 Free PMC article.
-
Ly6C- Monocytes Regulate Parasite-Induced Liver Inflammation by Inducing the Differentiation of Pathogenic Ly6C+ Monocytes into Macrophages.PLoS Pathog. 2015 May 28;11(5):e1004873. doi: 10.1371/journal.ppat.1004873. eCollection 2015 May. PLoS Pathog. 2015. PMID: 26020782 Free PMC article.
-
Recruitment of hepatic macrophages from monocytes is independent of IL-4Rα but is associated with ablation of resident macrophages in schistosomiasis.Eur J Immunol. 2019 Jul;49(7):1067-1081. doi: 10.1002/eji.201847796. Epub 2019 Apr 24. Eur J Immunol. 2019. PMID: 30919955
-
The Immune Function of Ly6Chi Inflammatory Monocytes During Infection and Inflammation.Curr Mol Med. 2017;17(1):4-12. doi: 10.2174/1566524017666170220102732. Curr Mol Med. 2017. PMID: 28231755 Review.
-
Monocytes and macrophages as cellular targets in liver fibrosis.Inflamm Allergy Drug Targets. 2009 Sep;8(4):307-18. doi: 10.2174/187152809789352230. Inflamm Allergy Drug Targets. 2009. PMID: 19534673 Review.
Cited by
-
The complex myeloid network of the liver with diverse functional capacity at steady state and in inflammation.Front Immunol. 2015 Apr 20;6:179. doi: 10.3389/fimmu.2015.00179. eCollection 2015. Front Immunol. 2015. PMID: 25941527 Free PMC article. Review.
-
Hepatic Macrophage Responses in Inflammation, a Function of Plasticity, Heterogeneity or Both?Front Immunol. 2021 Jun 9;12:690813. doi: 10.3389/fimmu.2021.690813. eCollection 2021. Front Immunol. 2021. PMID: 34177948 Free PMC article. Review.
-
Schistosome and intestinal helminth modulation of macrophage immunometabolism.Immunology. 2021 Feb;162(2):123-134. doi: 10.1111/imm.13231. Epub 2020 Jul 27. Immunology. 2021. PMID: 32614982 Free PMC article. Review.
-
Schistosome Infection Impacts Hematopoiesis.J Immunol. 2024 Feb 15;212(4):607-616. doi: 10.4049/jimmunol.2300195. J Immunol. 2024. PMID: 38169327 Free PMC article.
-
Role of LpL (Lipoprotein Lipase) in Macrophage Polarization In Vitro and In Vivo.Arterioscler Thromb Vasc Biol. 2019 Oct;39(10):1967-1985. doi: 10.1161/ATVBAHA.119.312389. Epub 2019 Aug 22. Arterioscler Thromb Vasc Biol. 2019. PMID: 31434492 Free PMC article.
References
-
- Fairfax K, Nascimento M, Huang SC, Everts B, Pearce EJ (2012) Th2 responses in schistosomiasis. Semin Immunopathol 34: 863–871. - PubMed
-
- Auffray C, Sieweke MH, Geissmann F (2009) Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol 27: 669–692. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

