A randomized, double-blind, placebo-controlled clinical trial evaluating Dermytol(®) cream for the treatment of actinic keratoses
- PMID: 25143750
- PMCID: PMC4132222
- DOI: 10.2147/CCID.S63067
A randomized, double-blind, placebo-controlled clinical trial evaluating Dermytol(®) cream for the treatment of actinic keratoses
Abstract
Purpose: Actinic keratosis lesions (AKs) have the potential to develop into squamous cell carcinoma (SCC) and thus therapies to prevent SCC development from AKs are warranted. The aim of this study was to assess the effects of a 3 month application of a canola phenolic acid-based cream (CPA) on AK lesions.
Patients and methods: This was a randomized, double-blind, placebo-controlled, 12 week clinical study conducted at a single-center in Santo Domingo, Dominican Republic. Forty-five subjects (30 CPA and 15 placebo), aged 45-85 years with 3-10 AKs within a 20 cm(2) treatment area (scalp, forehead, dorsal forearm, neck, or back of hand) were enrolled. The primary outcome was complete or partial lesion clearance and the secondary outcome was safety of CPA.
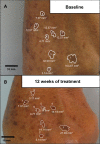
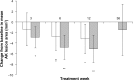
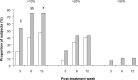
Results: Although complete AK lesion clearance was not seen in this study, a significant reduction in the mean change from baseline in the average lesion area was observed at weeks 3 (P=0.002), 6 (P<0.001), and 12 (P<0.001) in the CPA group, but only at weeks 6 and 12 in the placebo group (P=0.005 and P=0.002, respectively). Furthermore, the proportion of participants with a $10% decrease in average lesion area was significantly higher in the CPA group than the placebo group at weeks 3 (P=0.05) and 6 (P=0.02), and showed a trend at week 12 (P=0.06). A subset analysis of the change in average lesion area based upon the total lesion area at baseline revealed that CPA elicited a greater reduction than placebo (2×) in participants with a baseline total AK lesion area of 100-500 mm(2) than in participants with a total area <100 mm(2) (1.3×).
Conclusion: The results of this study and previous in vitro studies suggest a potential role for CPA in the treatment of AK lesions and the prevention of SCC development.
Keywords: Dermytol®; actinic keratosis; canola phenolic acid-rich extract; precancerous lesion; squamous cell carcinoma.
Figures
Similar articles
-
Results of a randomized, placebo-controlled safety and efficacy study of topical diclofenac 3% gel in organ transplant patients with multiple actinic keratoses.Eur J Dermatol. 2010 Jul-Aug;20(4):482-8. doi: 10.1684/ejd.2010.1010. Epub 2010 May 27. Eur J Dermatol. 2010. PMID: 20507841 Clinical Trial.
-
Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: two phase 3 multicenter, randomized, double-blind, placebo-controlled studies.J Drugs Dermatol. 2013 Nov;12(11):1278-82. J Drugs Dermatol. 2013. PMID: 24196337 Clinical Trial.
-
Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: two phase 3, multicenter, randomized, double-blind, placebo-controlled studies.J Drugs Dermatol. 2014 Feb;13(2):166-9. J Drugs Dermatol. 2014. PMID: 24509967 Clinical Trial.
-
One-week treatment with once-daily fluorouracil cream 0.5% in participants with actinic keratoses.Cutis. 2008 Jun;81(6):509-16. Cutis. 2008. PMID: 18666394 Review.
-
5-fluorouracil 0.5% cream for multiple actinic or solar keratoses of the face and anterior scalp.Skin Therapy Lett. 2001 Jun;6(9):1-4. Skin Therapy Lett. 2001. PMID: 11550079 Review.
References
-
- Marks R. Solar keratoses. Br J Dermatol. 1990;122(Suppl 35):49–54. - PubMed
-
- Marks R, Foley P, Goodman G, Hage BH, Selwood TS. Spontaneous remission of solar keratoses: the case for conservative management. Br J Dermatol. 1986;115(6):649–655. - PubMed
-
- Neidecker MV, Davis-Ajami ML, Balkrishnan R, Feldman SR. Pharmacoeconomic considerations in treating actinic keratosis. Pharmacoeconomics. 2009;27(6):451–464. - PubMed
-
- Feldman SR, Fleischer AB., Jr Progression of actinic keratosis to squamous cell carcinoma revisited: Clinical and treatment implications. Cutis. 2011;87(4):201–207. - PubMed
-
- Martin G, Swanson N. Clinical findings using ingenol mebutate gel to treat actinic keratoses. J Am Acad Dermatol. 2013;68(1 Suppl 1):S39–S48. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

