Humoral responses to independent vaccinations are correlated in healthy boosted adults
- PMID: 25140930
- PMCID: PMC4323156
- DOI: 10.1016/j.vaccine.2014.08.005
Humoral responses to independent vaccinations are correlated in healthy boosted adults
Abstract
Background: Roughly half of U.S. adults do not receive recommended booster vaccinations, but protective antibody levels are rarely measured in adults. Demographic factors, vaccination history, and responses to other vaccinations could help identify at-risk individuals. We sought to characterize rates of seroconversion and determine associations of humoral responses to multiple vaccinations in healthy adults.
Methods: Humoral responses toward measles, mumps, tetanus toxoid, pertussis, hepatitis B surface antigen, and anthrax protective antigen were measured by ELISA in post-immunization samples from 1465 healthy U.S. military members. We examined the effects of demographic and clinical factors on immunization responses, as well as assessed correlations between vaccination responses.
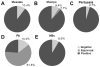
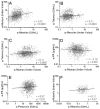
Results: Subsets of boosted adults did not have seroprotective levels of antibodies toward measles (10.4%), mumps (9.4%), pertussis (4.7%), hepatitis B (8.6%) or protective antigen (14.4%) detected. Half-lives of antibody responses were generally long (>30 years). Measles and mumps antibody levels were correlated (r=0.31, p<0.001), but not associated with select demographic features or vaccination history. Measles and mumps antibody levels also correlated with tetanus antibody response (r=0.11, p<0.001).
Conclusions: Vaccination responses are predominantly robust and vaccine specific. However, a small but significant portion of the vaccinated adult population may not have quantitative seroprotective antibody to common vaccine-preventable infections.
Keywords: Anthrax vaccine adsorbed; Hepatitis B; Measles; Mumps; Pertussis; Tetanus.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
S.R. Crowe is now affiliated with PharmAthene, Inc; however, her work with this study was only completed during her time at OMRF. All other authors declare that there are no conflicts of interest.
Figures

Similar articles
-
Assessment of humoral immunity to poliomyelitis, tetanus, hepatitis B, measles, rubella, and mumps in children after chemotherapy.Cancer. 2004 Aug 1;101(3):635-41. doi: 10.1002/cncr.20384. Cancer. 2004. PMID: 15274078
-
A phase III, open-label, randomised multicentre study to evaluate the immunogenicity and safety of a booster dose of two different reduced antigen diphtheria-tetanus-acellular pertussis-polio vaccines, when co-administered with measles-mumps-rubella vaccine in 3 and 4-year-old healthy children in the UK.Vaccine. 2018 Apr 19;36(17):2300-2306. doi: 10.1016/j.vaccine.2018.03.021. Epub 2018 Mar 22. Vaccine. 2018. PMID: 29576304 Clinical Trial.
-
Seroprevalences of antibodies against pertussis, diphtheria, tetanus, measles, mumps and rubella: A cross-sectional study in children following vaccination procedure in Guangzhou, China.Vaccine. 2020 May 13;38(23):3960-3967. doi: 10.1016/j.vaccine.2020.03.056. Epub 2020 Apr 19. Vaccine. 2020. PMID: 32321685
-
Reduced-antigen, combined diphtheria, tetanus and acellular pertussis vaccine, adsorbed (Boostrix®): a review of its properties and use as a single-dose booster immunization.Drugs. 2012 Sep 10;72(13):1765-91. doi: 10.2165/11209630-000000000-00000. Drugs. 2012. PMID: 22931522 Review.
-
Antibody responses of healthy infants to concurrent administration of a bivalent haemophilus influenzae type b-hepatitis B vaccine with diphtheria-tetanus-pertussis, polio and measles-mumps-rubella vaccines.BioDrugs. 2001;15(6):413-8. doi: 10.2165/00063030-200115060-00007. BioDrugs. 2001. PMID: 11520252 Review.
Cited by
-
Incidence of Tetanus and Diphtheria in Relation to Adult Vaccination Schedules.Clin Infect Dis. 2021 Jan 27;72(2):285-292. doi: 10.1093/cid/ciaa017. Clin Infect Dis. 2021. PMID: 32095828 Free PMC article.
-
Lethal factor antibodies contribute to lethal toxin neutralization in recipients of anthrax vaccine precipitated.Vaccine. 2017 Jun 8;35(26):3416-3422. doi: 10.1016/j.vaccine.2017.05.006. Epub 2017 May 11. Vaccine. 2017. PMID: 28504191 Free PMC article.
-
Durability of Vaccine-Induced Immunity Against Tetanus and Diphtheria Toxins: A Cross-sectional Analysis.Clin Infect Dis. 2016 May 1;62(9):1111-1118. doi: 10.1093/cid/ciw066. Epub 2016 Mar 21. Clin Infect Dis. 2016. PMID: 27060790 Free PMC article.
-
Anthrax Vaccine Precipitated Induces Edema Toxin-Neutralizing, Edema Factor-Specific Antibodies in Human Recipients.Clin Vaccine Immunol. 2017 Nov 6;24(11):e00165-17. doi: 10.1128/CVI.00165-17. Print 2017 Nov. Clin Vaccine Immunol. 2017. PMID: 28877928 Free PMC article.
References
-
- Shapiro ED. Acellular vaccines and resurgence of pertussis. JAMA. 2012;308:2149–50. - PubMed
-
- Cherry JD. The epidemiology of pertussis: a comparison of the epidemiology of the disease pertussis with the epidemiology of Bordetella pertussis infection. Pediatrics. 2005;115:1422–7. - PubMed
-
- Centers for Disease C and Prevention. Advisory Committee on Immunization Practices (ACIP) recommended immunization schedules for persons aged 0 through 18 years and adults aged 19 years and older--United States, 2013. Morbidity and mortality weekly report Surveillance summaries. 2013;62 (Suppl 1):1. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

