S-acylation dependent post-translational cross-talk regulates large conductance calcium- and voltage- activated potassium (BK) channels
- PMID: 25140154
- PMCID: PMC4122160
- DOI: 10.3389/fphys.2014.00281
S-acylation dependent post-translational cross-talk regulates large conductance calcium- and voltage- activated potassium (BK) channels
Abstract
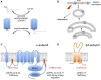
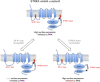
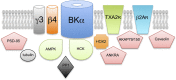
Mechanisms that control surface expression and/or activity of large conductance calcium-activated potassium (BK) channels are important determinants of their (patho)physiological function. Indeed, BK channel dysfunction is associated with major human disorders ranging from epilepsy to hypertension and obesity. S-acylation (S-palmitoylation) represents a major reversible, post-translational modification controlling the properties and function of many proteins including ion channels. Recent evidence reveals that both pore-forming and regulatory subunits of BK channels are S-acylated and control channel trafficking and regulation by AGC-family protein kinases. The pore-forming α-subunit is S-acylated at two distinct sites within the N- and C-terminus, each site being regulated by different palmitoyl acyl transferases (zDHHCs) and acyl thioesterases (APTs). S-acylation of the N-terminus controls channel trafficking and surface expression whereas S-acylation of the C-terminal domain determines regulation of channel activity by AGC-family protein kinases. S-acylation of the regulatory β4-subunit controls ER exit and surface expression of BK channels but does not affect ion channel kinetics at the plasma membrane. Furthermore, a significant number of previously identified BK-channel interacting proteins have been shown, or are predicted to be, S-acylated. Thus, the BK channel multi-molecular signaling complex may be dynamically regulated by this fundamental post-translational modification and thus S-acylation likely represents an important determinant of BK channel physiology in health and disease.
Keywords: KCNMA1; KCNMB4; MaxiK channel; Slo1; acylation; palmitoylation; phosphorylation; trafficking.
Figures
Similar articles
-
Regulation of large conductance calcium- and voltage-activated potassium (BK) channels by S-palmitoylation.Biochem Soc Trans. 2013 Feb 1;41(1):67-71. doi: 10.1042/BST20120226. Biochem Soc Trans. 2013. PMID: 23356260 Free PMC article.
-
S-Acylation controls functional coupling of BK channel pore-forming α-subunits and β1-subunits.J Biol Chem. 2019 Aug 9;294(32):12066-12076. doi: 10.1074/jbc.RA119.009065. Epub 2019 Jun 18. J Biol Chem. 2019. PMID: 31213527 Free PMC article.
-
Palmitoylation of the β4-subunit regulates surface expression of large conductance calcium-activated potassium channel splice variants.J Biol Chem. 2013 May 3;288(18):13136-44. doi: 10.1074/jbc.M113.461830. Epub 2013 Mar 16. J Biol Chem. 2013. PMID: 23504458 Free PMC article.
-
Posttranscriptional and Posttranslational Regulation of BK Channels.Int Rev Neurobiol. 2016;128:91-126. doi: 10.1016/bs.irn.2016.02.012. Epub 2016 Mar 3. Int Rev Neurobiol. 2016. PMID: 27238262 Review.
-
Current understanding of iberiotoxin-resistant BK channels in the nervous system.Front Physiol. 2014 Oct 9;5:382. doi: 10.3389/fphys.2014.00382. eCollection 2014. Front Physiol. 2014. PMID: 25346692 Free PMC article. Review.
Cited by
-
Palmitoylation of Voltage-Gated Ion Channels.Int J Mol Sci. 2022 Aug 19;23(16):9357. doi: 10.3390/ijms23169357. Int J Mol Sci. 2022. PMID: 36012639 Free PMC article. Review.
-
An unexpected journey: conceptual evolution of mechanoregulated potassium transport in the distal nephron.Am J Physiol Cell Physiol. 2016 Feb 15;310(4):C243-59. doi: 10.1152/ajpcell.00328.2015. Epub 2015 Dec 2. Am J Physiol Cell Physiol. 2016. PMID: 26632600 Free PMC article. Review.
-
Oxidative Stress and Maxi Calcium-Activated Potassium (BK) Channels.Biomolecules. 2015 Aug 17;5(3):1870-911. doi: 10.3390/biom5031870. Biomolecules. 2015. PMID: 26287261 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

