BB0323 and novel virulence determinant BB0238: Borrelia burgdorferi proteins that interact with and stabilize each other and are critical for infectivity
- PMID: 25139020
- PMCID: PMC4351374
- DOI: 10.1093/infdis/jiu460
BB0323 and novel virulence determinant BB0238: Borrelia burgdorferi proteins that interact with and stabilize each other and are critical for infectivity
Abstract
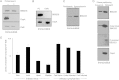
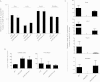
We have shown that Borrelia burgdorferi gene product BB0323 is essential for cell fission and pathogen persistence in vivo. Here we describe characterization of a conserved hypothetical protein annotated as BB0238, which specifically interacts with the N-terminal region of BB0323. We show that BB0238 is a subsurface protein, and similar to BB0323, exists in the periplasm and as a membrane-bound protein. Deletion of bb0238 in infectious B. burgdorferi did not affect microbial growth in vitro or survival in ticks, but the mutant was unable to persist in mice or transmit from ticks--defects that are restored on genetic complementation. Remarkably, BB0238 and BB0323 contribute to mutual posttranslational stability, because deletion of one causes dramatic reduction in the protein level of the other partner. Interference with the function of BB0238 or BB0323 and their interaction may provide novel strategies to combat B. burgdorferi infection.
Keywords: BB0238; BB0323; Borrelia burgdorferi; pathogen persistence; posttranslational stability; protein–protein interaction.
© The Author 2014. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures



Similar articles
-
A protein-protein interaction dictates Borrelial infectivity.Sci Rep. 2017 Jun 7;7(1):2932. doi: 10.1038/s41598-017-03279-7. Sci Rep. 2017. PMID: 28592866 Free PMC article.
-
BB0238, a presumed tetratricopeptide repeat-containing protein, is required during Borrelia burgdorferi mammalian infection.Infect Immun. 2014 Oct;82(10):4292-306. doi: 10.1128/IAI.01977-14. Epub 2014 Jul 28. Infect Immun. 2014. PMID: 25069985 Free PMC article.
-
BB0323 function is essential for Borrelia burgdorferi virulence and persistence through tick-rodent transmission cycle.J Infect Dis. 2009 Oct 15;200(8):1318-30. doi: 10.1086/605846. J Infect Dis. 2009. PMID: 19754308 Free PMC article.
-
Borrelia burgdorferi protein interactions critical for microbial persistence in mammals.Cell Microbiol. 2019 Feb;21(2):e12885. doi: 10.1111/cmi.12885. Epub 2018 Jul 8. Cell Microbiol. 2019. PMID: 29934966 Free PMC article. Review.
-
The Lyme disease spirochete, Borrelia burgdorferi, as a model vector-borne pathogen: insights on regulation of gene and protein expression.Curr Opin Microbiol. 2023 Aug;74:102332. doi: 10.1016/j.mib.2023.102332. Epub 2023 Jun 4. Curr Opin Microbiol. 2023. PMID: 37279610 Free PMC article. Review.
Cited by
-
HtrA, a Temperature- and Stationary Phase-Activated Protease Involved in Maturation of a Key Microbial Virulence Determinant, Facilitates Borrelia burgdorferi Infection in Mammalian Hosts.Infect Immun. 2016 Jul 21;84(8):2372-2381. doi: 10.1128/IAI.00360-16. Print 2016 Aug. Infect Immun. 2016. PMID: 27271745 Free PMC article.
-
Lyme Disease Pathogenesis.Curr Issues Mol Biol. 2021;42:473-518. doi: 10.21775/cimb.042.473. Epub 2020 Dec 23. Curr Issues Mol Biol. 2021. PMID: 33353871 Free PMC article. Review.
-
Dome1-JAK-STAT signaling between parasite and host integrates vector immunity and development.Science. 2023 Jan 13;379(6628):eabl3837. doi: 10.1126/science.abl3837. Epub 2023 Jan 13. Science. 2023. PMID: 36634189 Free PMC article.
-
Characterization of the Flagellar Collar Reveals Structural Plasticity Essential for Spirochete Motility.mBio. 2021 Dec 21;12(6):e0249421. doi: 10.1128/mBio.02494-21. Epub 2021 Nov 23. mBio. 2021. PMID: 34809456 Free PMC article.
-
Serologic Evidence for the Exposure of Eastern Coyotes (Canis latrans) in Pennsylvania to the Tick-Borne Pathogens Borreliella burgdorferi and Anaplasma phagocytophilum.mSphere. 2020 Aug 12;5(4):e00544-20. doi: 10.1128/mSphere.00544-20. mSphere. 2020. PMID: 32817454 Free PMC article.
References
-
- Piesman J, Eisen L. Prevention of tick-borne diseases. Annu Rev Entomol. 2008;53:323–43. - PubMed
-
- Piesman J, Gern L. Lyme borreliosis in Europe and North America. Parasitology. 2004;129(suppl)):S191–220. - PubMed
-
- Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease—a tick-borne spirochetosis? Science. 1982;216:1317–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

