A unique PDZ domain and arrestin-like fold interaction reveals mechanistic details of endocytic recycling by SNX27-retromer
- PMID: 25136126
- PMCID: PMC4156734
- DOI: 10.1073/pnas.1410552111
A unique PDZ domain and arrestin-like fold interaction reveals mechanistic details of endocytic recycling by SNX27-retromer
Abstract
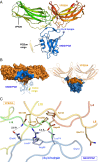
The sorting nexin 27 (SNX27)-retromer complex is a major regulator of endosome-to-plasma membrane recycling of transmembrane cargos that contain a PSD95, Dlg1, zo-1 (PDZ)-binding motif. Here we describe the core interaction in SNX27-retromer assembly and its functional relevance for cargo sorting. Crystal structures and NMR experiments reveal that an exposed β-hairpin in the SNX27 PDZ domain engages a groove in the arrestin-like structure of the vacuolar protein sorting 26A (VPS26A) retromer subunit. The structure establishes how the SNX27 PDZ domain simultaneously binds PDZ-binding motifs and retromer-associated VPS26. Importantly, VPS26A binding increases the affinity of the SNX27 PDZ domain for PDZ- binding motifs by an order of magnitude, revealing cooperativity in cargo selection. With disruption of SNX27 and retromer function linked to synaptic dysfunction and neurodegenerative disease, our work provides the first step, to our knowledge, in the molecular description of this important sorting complex, and more broadly describes a unique interaction between a PDZ domain and an arrestin-like fold.
Keywords: Alzheimer's disease; Down syndrome; Parkinson disease; endosomal recycling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


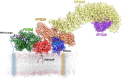
Similar articles
-
Actin-Sorting Nexin 27 (SNX27)-Retromer Complex Mediates Rapid Parathyroid Hormone Receptor Recycling.J Biol Chem. 2016 May 20;291(21):10986-1002. doi: 10.1074/jbc.M115.697045. Epub 2016 Mar 23. J Biol Chem. 2016. PMID: 27008860 Free PMC article.
-
Regulation of the endosomal SNX27-retromer by OTULIN.Nat Commun. 2019 Sep 20;10(1):4320. doi: 10.1038/s41467-019-12309-z. Nat Commun. 2019. PMID: 31541095 Free PMC article.
-
A global analysis of SNX27-retromer assembly and cargo specificity reveals a function in glucose and metal ion transport.Nat Cell Biol. 2013 May;15(5):461-71. doi: 10.1038/ncb2721. Epub 2013 Apr 7. Nat Cell Biol. 2013. PMID: 23563491 Free PMC article.
-
Retromer and sorting nexins in endosomal sorting.Biochem Soc Trans. 2015 Feb;43(1):33-47. doi: 10.1042/BST20140290. Biochem Soc Trans. 2015. PMID: 25619244 Review.
-
Toward Understanding the Molecular Role of SNX27/Retromer in Human Health and Disease.Front Cell Dev Biol. 2021 Apr 15;9:642378. doi: 10.3389/fcell.2021.642378. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33937239 Free PMC article. Review.
Cited by
-
Mechanism and regulation of cargo entry into the Commander endosomal recycling pathway.Nat Commun. 2024 Aug 21;15(1):7180. doi: 10.1038/s41467-024-50971-0. Nat Commun. 2024. PMID: 39168982 Free PMC article.
-
Cargo adaptors: structures illuminate mechanisms regulating vesicle biogenesis.Trends Cell Biol. 2015 Jul;25(7):408-16. doi: 10.1016/j.tcb.2015.02.005. Epub 2015 Mar 17. Trends Cell Biol. 2015. PMID: 25795254 Free PMC article. Review.
-
A molecular code for endosomal recycling of phosphorylated cargos by the SNX27-retromer complex.Nat Struct Mol Biol. 2016 Oct;23(10):921-932. doi: 10.1038/nsmb.3290. Epub 2016 Sep 5. Nat Struct Mol Biol. 2016. PMID: 27595347
-
Cholera toxin inhibits SNX27-retromer-mediated delivery of cargo proteins to the plasma membrane.J Cell Sci. 2018 Aug 17;131(16):jcs218610. doi: 10.1242/jcs.218610. J Cell Sci. 2018. PMID: 30030371 Free PMC article.
-
Deep learning-based identification of genetic variants: application to Alzheimer's disease classification.Brief Bioinform. 2022 Mar 10;23(2):bbac022. doi: 10.1093/bib/bbac022. Brief Bioinform. 2022. PMID: 35183061 Free PMC article.
References
-
- Johannes L, Popoff V. Tracing the retrograde route in protein trafficking. Cell. 2008;135(7):1175–1187. - PubMed
-
- Hsu VW, Bai M, Li J. Getting active: Protein sorting in endocytic recycling. Nat Rev Mol Cell Biol. 2012;13(5):323–328. - PubMed
-
- Collins BM, Skinner CF, Watson PJ, Seaman MN, Owen DJ. Vps29 has a phosphoesterase fold that acts as a protein interaction scaffold for retromer assembly. Nat Struct Mol Biol. 2005;12(7):594–602. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

