A pro-inflammatory role for Th22 cells in Helicobacter pylori-associated gastritis
- PMID: 25134787
- PMCID: PMC4552937
- DOI: 10.1136/gutjnl-2014-307020
A pro-inflammatory role for Th22 cells in Helicobacter pylori-associated gastritis
Abstract
Objective: Helper T (Th) cell responses are critical for the pathogenesis of Helicobacter pylori-induced gastritis. Th22 cells represent a newly discovered Th cell subset, but their relevance to H. pylori-induced gastritis is unknown.
Design: Flow cytometry, real-time PCR and ELISA analyses were performed to examine cell, protein and transcript levels in gastric samples from patients and mice infected with H. pylori. Gastric tissues from interleukin (IL)-22-deficient and wild-type (control) mice were also examined. Tissue inflammation was determined for pro-inflammatory cell infiltration and pro-inflammatory protein production. Gastric epithelial cells and myeloid-derived suppressor cells (MDSC) were isolated, stimulated and/or cultured for Th22 cell function assays.
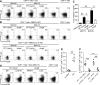
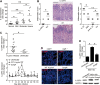
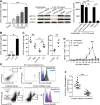
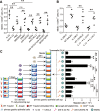
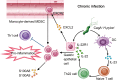
Results: Th22 cells accumulated in gastric mucosa of both patients and mice infected with H. pylori. Th22 cell polarisation was promoted via the production of IL-23 by dendritic cells (DC) during H. pylori infection, and resulted in increased inflammation within the gastric mucosa. This inflammation was characterised by the CXCR2-dependent influx of MDSCs, whose migration was induced via the IL-22-dependent production of CXCL2 by gastric epithelial cells. Under the influence of IL-22, MDSCs, in turn, produced pro-inflammatory proteins, such as S100A8 and S100A9, and suppressed Th1 cell responses, thereby contributing to the development of H. pylori-associated gastritis.
Conclusions: This study, therefore, identifies a novel regulatory network involving H. pylori, DCs, Th22 cells, gastric epithelial cells and MDSCs, which collectively exert a pro-inflammatory effect within the gastric microenvironment. Efforts to inhibit this Th22-dependent pathway may therefore prove a valuable strategy in the therapy of H. pylori-associated gastritis.
Keywords: GASTRITIS; HELICOBACTER PYLORI.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures


Similar articles
-
Helicobacter pylori-induced REDD1 modulates Th17 cell responses that contribute to gastritis.Clin Sci (Lond). 2021 Nov 26;135(22):2541-2558. doi: 10.1042/CS20210753. Clin Sci (Lond). 2021. PMID: 34730176
-
Role of Th22 cells in Helicobacter pylori-related gastritis and peptic ulcer diseases.Mol Biol Rep. 2019 Dec;46(6):5703-5712. doi: 10.1007/s11033-019-05004-1. Epub 2019 Jul 29. Mol Biol Rep. 2019. PMID: 31359381
-
GITR Promotes the Polarization of TFH-Like Cells in Helicobacter pylori-Positive Gastritis.Front Immunol. 2021 Sep 10;12:736269. doi: 10.3389/fimmu.2021.736269. eCollection 2021. Front Immunol. 2021. PMID: 34589088 Free PMC article.
-
Impairment of ghrelin synthesis in Helicobacter pylori-colonized stomach: new clues for the pathogenesis of H. pylori-related gastric inflammation.World J Gastroenterol. 2014 Jan 21;20(3):639-46. doi: 10.3748/wjg.v20.i3.639. World J Gastroenterol. 2014. PMID: 24574737 Free PMC article. Review.
-
Helicobacter pylori and gastric inflammation.Br Med Bull. 1998;54(1):139-50. doi: 10.1093/oxfordjournals.bmb.a011664. Br Med Bull. 1998. PMID: 9604438 Review.
Cited by
-
Th17, Th22, and Myeloid-Derived Suppressor Cell Population Dynamics and Response to IL-6 in 4T1 Mammary Carcinoma.Int J Mol Sci. 2022 Sep 7;23(18):10299. doi: 10.3390/ijms231810299. Int J Mol Sci. 2022. PMID: 36142210 Free PMC article.
-
Cytokines IL-17 and IL-22 in the host response to infection.Pathog Dis. 2016 Dec;74(9):ftw111. doi: 10.1093/femspd/ftw111. Epub 2016 Dec 2. Pathog Dis. 2016. PMID: 27915228 Free PMC article. Review.
-
Treatment with Cestode Parasite Antigens Results in Recruitment of CCR2+ Myeloid Cells, the Adoptive Transfer of Which Ameliorates Colitis.Infect Immun. 2016 Nov 18;84(12):3471-3483. doi: 10.1128/IAI.00681-16. Print 2016 Dec. Infect Immun. 2016. PMID: 27672083 Free PMC article.
-
Differential inflammatory response to Helicobacter pylori infection: etiology and clinical outcomes.J Inflamm Res. 2015 Aug 13;8:137-47. doi: 10.2147/JIR.S64888. eCollection 2015. J Inflamm Res. 2015. PMID: 26316793 Free PMC article. Review.
-
Helicobacter pylori infection has a detrimental impact on the efficacy of cancer immunotherapies.Gut. 2022 Mar;71(3):457-466. doi: 10.1136/gutjnl-2020-323392. Epub 2021 Jul 12. Gut. 2022. PMID: 34253574 Free PMC article.
References
-
- Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med 2002;347:1175–86. - PubMed
-
- Eaton KA, Mefford M, Thevenot T. The role of T cell subsets and cytokines in the pathogenesis of Helicobacter pylori gastritis in mice. J Immunol 2001;166:7456–61. - PubMed
-
- Radaeva S, Sun R, Pan HN, et al. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology 2004;39:1332–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
