Alzheimer's disease: relevant molecular and physiopathological events affecting amyloid-β brain balance and the putative role of PPARs
- PMID: 25120477
- PMCID: PMC4112937
- DOI: 10.3389/fnagi.2014.00176
Alzheimer's disease: relevant molecular and physiopathological events affecting amyloid-β brain balance and the putative role of PPARs
Abstract
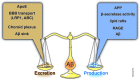
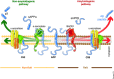
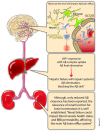
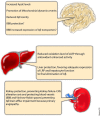
Alzheimer's disease (AD) is the most common form of age-related dementia. With the expected aging of the human population, the estimated morbidity of AD suggests a critical upcoming health problem. Several lines of research are focused on understanding AD pathophysiology, and although the etiology of the disease remains a matter of intense debate, increased brain levels of amyloid-β (Aβ) appear to be a critical event in triggering a wide range of molecular alterations leading to AD. It has become evident in recent years that an altered balance between production and clearance is responsible for the accumulation of brain Aβ. Moreover, Aβ clearance is a complex event that involves more than neurons and microglia. The status of the blood-brain barrier (BBB) and choroid plexus, along with hepatic functionality, should be considered when Aβ balance is addressed. Furthermore, it has been proposed that exposure to sub-toxic concentrations of metals, such as copper, could both directly affect these secondary structures and act as a seeding or nucleation core that facilitates Aβ aggregation. Recently, we have addressed peroxisomal proliferator-activated receptors (PPARs)-related mechanisms, including the direct modulation of mitochondrial dynamics through the PPARγ-coactivator-1α (PGC-1α) axis and the crosstalk with critical aging- and neurodegenerative-related cellular pathways. In the present review, we revise the current knowledge regarding the molecular aspects of Aβ production and clearance and provide a physiological context that gives a more complete view of this issue. Additionally, we consider the different structures involved in AD-altered Aβ brain balance, which could be directly or indirectly affected by a nuclear receptor (NR)/PPAR-related mechanism.
Keywords: Aβ balance; blood-brain barrier; brain homeostasis; neurodegenerative disorders; nuclear receptors; systemic Aβ clearance.
Figures
Similar articles
-
Clearance of amyloid-β peptide across the choroid plexus in Alzheimer's disease.Curr Aging Sci. 2010 Dec;3(3):219-29. doi: 10.2174/1874609811003030219. Curr Aging Sci. 2010. PMID: 20735345 Review.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
In vivo Differential Brain Clearance and Catabolism of Monomeric and Oligomeric Alzheimer's Aβ protein.Front Aging Neurosci. 2016 Sep 27;8:223. doi: 10.3389/fnagi.2016.00223. eCollection 2016. Front Aging Neurosci. 2016. PMID: 27729857 Free PMC article.
-
Astrocytic LRP1 Mediates Brain Aβ Clearance and Impacts Amyloid Deposition.J Neurosci. 2017 Apr 12;37(15):4023-4031. doi: 10.1523/JNEUROSCI.3442-16.2017. Epub 2017 Mar 8. J Neurosci. 2017. PMID: 28275161 Free PMC article.
-
Peroxisome proliferator-activated receptors and Alzheimer's disease: hitting the blood-brain barrier.Mol Neurobiol. 2013 Dec;48(3):438-51. doi: 10.1007/s12035-013-8435-5. Epub 2013 Mar 14. Mol Neurobiol. 2013. PMID: 23494748 Review.
Cited by
-
Is Alzheimer's Disease a Liver Disease of the Brain?J Alzheimers Dis. 2020;75(1):1-14. doi: 10.3233/JAD-190848. J Alzheimers Dis. 2020. PMID: 32250293 Free PMC article. Review.
-
CALHM1 ion channel elicits amyloid-β clearance by insulin-degrading enzyme in cell lines and in vivo in the mouse brain.J Cell Sci. 2015 Jul 1;128(13):2330-8. doi: 10.1242/jcs.167270. Epub 2015 May 21. J Cell Sci. 2015. PMID: 25999473 Free PMC article.
-
Peroxisome turnover and diurnal modulation of antioxidant activity in retinal pigment epithelia utilizes microtubule-associated protein 1 light chain 3B (LC3B).Am J Physiol Cell Physiol. 2019 Dec 1;317(6):C1194-C1204. doi: 10.1152/ajpcell.00185.2019. Epub 2019 Oct 2. Am J Physiol Cell Physiol. 2019. PMID: 31577510 Free PMC article.
-
Ginsenoside Rg1 alleviates learning and memory impairments and Aβ disposition through inhibiting NLRP1 inflammasome and autophagy dysfunction in APP/PS1 mice.Mol Med Rep. 2023 Jan;27(1):6. doi: 10.3892/mmr.2022.12893. Epub 2022 Nov 11. Mol Med Rep. 2023. PMID: 36367174 Free PMC article.
-
Dendrobium nobile Lindl. Alkaloids Decreases the Level of Intracellular β-Amyloid by Improving Impaired Autolysosomal Proteolysis in APP/PS1 Mice.Front Pharmacol. 2018 Dec 18;9:1479. doi: 10.3389/fphar.2018.01479. eCollection 2018. Front Pharmacol. 2018. PMID: 30618767 Free PMC article.
References
-
- Aleshin S., Strokin M., Sergeeva M., Reiser G. (2013). Peroxisome proliferator activated receptor (PPAR)β/δ, a possible nexus of PPARα- and PPARγ-dependent molecular pathways in neurodegenerative diseases: review and novel hypotheses. Neurochem. Int. 63, 322–330 10.1016/j.neuint.2013.06.012 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

