A molecular web: endoplasmic reticulum stress, inflammation, and oxidative stress
- PMID: 25120434
- PMCID: PMC4114208
- DOI: 10.3389/fncel.2014.00213
A molecular web: endoplasmic reticulum stress, inflammation, and oxidative stress
Abstract
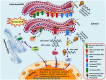
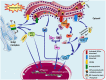
Execution of fundamental cellular functions demands regulated protein folding homeostasis. Endoplasmic reticulum (ER) is an active organelle existing to implement this function by folding and modifying secretory and membrane proteins. Loss of protein folding homeostasis is central to various diseases and budding evidences suggest ER stress as being a major contributor in the development or pathology of a diseased state besides other cellular stresses. The trigger for diseases may be diverse but, inflammation and/or ER stress may be basic mechanisms increasing the severity or complicating the condition of the disease. Chronic ER stress and activation of the unfolded-protein response (UPR) through endogenous or exogenous insults may result in impaired calcium and redox homeostasis, oxidative stress via protein overload thereby also influencing vital mitochondrial functions. Calcium released from the ER augments the production of mitochondrial Reactive Oxygen Species (ROS). Toxic accumulation of ROS within ER and mitochondria disturbs fundamental organelle functions. Sustained ER stress is known to potentially elicit inflammatory responses via UPR pathways. Additionally, ROS generated through inflammation or mitochondrial dysfunction could accelerate ER malfunction. Dysfunctional UPR pathways have been associated with a wide range of diseases including several neurodegenerative diseases, stroke, metabolic disorders, cancer, inflammatory disease, diabetes mellitus, cardiovascular disease, and others. In this review, we have discussed the UPR signaling pathways, and networking between ER stress-induced inflammatory pathways, oxidative stress, and mitochondrial signaling events, which further induce or exacerbate ER stress.
Keywords: IRE1α; NF-κB; calcium; endoplasmic reticulum stress; inflammation; oxidative stress.
Figures

Similar articles
-
Cancer and ER stress: Mutual crosstalk between autophagy, oxidative stress and inflammatory response.Biomed Pharmacother. 2019 Oct;118:109249. doi: 10.1016/j.biopha.2019.109249. Epub 2019 Jul 24. Biomed Pharmacother. 2019. PMID: 31351428 Review.
-
Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease.Antioxid Redox Signal. 2014 Jul 20;21(3):396-413. doi: 10.1089/ars.2014.5851. Epub 2014 Jun 12. Antioxid Redox Signal. 2014. PMID: 24702237 Free PMC article. Review.
-
The effects of induced production of reactive oxygen species in organelles on endoplasmic reticulum stress and on the unfolded protein response in arabidopsis.Ann Bot. 2015 Sep;116(4):541-53. doi: 10.1093/aob/mcv072. Epub 2015 Jun 12. Ann Bot. 2015. PMID: 26070642 Free PMC article.
-
Endoplasmic reticulum stress and the unfolded protein responses in retinal degeneration.Exp Eye Res. 2014 Aug;125:30-40. doi: 10.1016/j.exer.2014.04.015. Epub 2014 May 2. Exp Eye Res. 2014. PMID: 24792589 Free PMC article. Review.
-
Unraveling the interplay between vital organelle stress and oxidative stress in idiopathic pulmonary fibrosis.Biochim Biophys Acta Mol Cell Res. 2024 Mar;1871(3):119676. doi: 10.1016/j.bbamcr.2024.119676. Epub 2024 Jan 17. Biochim Biophys Acta Mol Cell Res. 2024. PMID: 38242330 Review.
Cited by
-
NF-κB and its crosstalk with endoplasmic reticulum stress in atherosclerosis.Front Cardiovasc Med. 2022 Sep 20;9:988266. doi: 10.3389/fcvm.2022.988266. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36204587 Free PMC article. Review.
-
Degradation of polyomavirus JC T-antigen by stress involves the LIP isoform of C/EBP.Cell Cycle. 2015;14(13):2075-9. doi: 10.1080/15384101.2015.1042631. Epub 2015 May 27. Cell Cycle. 2015. PMID: 26017382 Free PMC article.
-
RNA-seq Characterization of Melanoma Phenotype Switch in 3D Collagen after p38 MAPK Inhibitor Treatment.Biomolecules. 2021 Mar 17;11(3):449. doi: 10.3390/biom11030449. Biomolecules. 2021. PMID: 33802847 Free PMC article.
-
Intracerebral Hemorrhage, Oxidative Stress, and Antioxidant Therapy.Oxid Med Cell Longev. 2016;2016:1203285. doi: 10.1155/2016/1203285. Epub 2016 Apr 14. Oxid Med Cell Longev. 2016. PMID: 27190572 Free PMC article. Review.
-
Glycated LDL increase VCAM-1 expression and secretion in endothelial cells and promote monocyte adhesion through mechanisms involving endoplasmic reticulum stress.Mol Cell Biochem. 2016 Jun;417(1-2):169-79. doi: 10.1007/s11010-016-2724-z. Epub 2016 May 20. Mol Cell Biochem. 2016. PMID: 27206739
References
-
- Alberts B., Johnson A., Lewis J., Raff M., Roberts K., Walter P. (2002). Molecular Biology of the Cell. New York: Garland Science
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

