PD-1 as an emerging therapeutic target in renal cell carcinoma: current evidence
- PMID: 25114573
- PMCID: PMC4122552
- DOI: 10.2147/OTT.S48443
PD-1 as an emerging therapeutic target in renal cell carcinoma: current evidence
Abstract
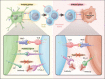
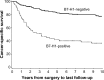
Renal cell carcinoma (RCC) is the most common primary malignant tumor of the kidney in adults, representing approximately 4% of all adult cancers in the United States. Metastatic RCC is poorly responsive to conventional cytotoxic chemotherapies but can be sensitive to T-cell-directed immunotherapies such as interferon-α or interleukin-2. Despite recent progress in the application of antiangiogenic "targeted therapies" for metastatic RCC, high-dose interleukin-2 remains an appropriate first-line therapy for select patients and is associated with durable complete remissions in a small fraction of treated patients. Thus, advanced RCC provides a unique opportunity to investigate the requirements for effective antitumor immunotherapy. Accumulating evidence suggests that resistance mechanisms exploited by RCC and other tumor types may play a dominant role in limiting the effectiveness of tumor-reactive adaptive immune responses. Expression of the inhibitory coreceptor programmed cell death-1 (PD-1) on tumor-infiltrating lymphocytes within RCC tumors, as well as the expression of the PD-1 ligand (PD-L1) on RCC tumor cells, are strong negative prognostic markers for disease-specific death in RCC patients. Monoclonal antibodies targeting either PD-1 or PD-L1 have now entered clinic trials and have demonstrated promising antitumor effects for refractory metastatic RCC. This review summarizes the results of published and reported studies of PD-1- and PD-L1-targeted therapies enrolling patients with advanced RCC, focusing on key safety, toxicity, and efficacy end points. Prospects for advanced phase clinical testing and novel therapy combinations with PD-1- and PD-L1-targeted agents are discussed.
Keywords: PD-1; PD-L1; T-lymphocyte; immune checkpoint; immunotherapy; renal cell carcinoma.
Figures
Similar articles
-
Targeting PD-1/PD-L1 in the treatment of metastatic renal cell carcinoma.Ther Adv Urol. 2015 Dec;7(6):365-77. doi: 10.1177/1756287215597647. Ther Adv Urol. 2015. PMID: 26622321 Free PMC article. Review.
-
Anti-PD-L1 treatment enhances antitumor effect of everolimus in a mouse model of renal cell carcinoma.Cancer Sci. 2016 Dec;107(12):1736-1744. doi: 10.1111/cas.13099. Epub 2016 Dec 13. Cancer Sci. 2016. PMID: 27712020 Free PMC article.
-
Association of Survival and Immune-Related Biomarkers With Immunotherapy in Patients With Non-Small Cell Lung Cancer: A Meta-analysis and Individual Patient-Level Analysis.JAMA Netw Open. 2019 Jul 3;2(7):e196879. doi: 10.1001/jamanetworkopen.2019.6879. JAMA Netw Open. 2019. PMID: 31290993 Free PMC article.
-
Clinical potential of PD-1/PD-L1 blockade therapy for renal cell carcinoma (RCC): a rapidly evolving strategy.Cancer Cell Int. 2022 Dec 12;22(1):401. doi: 10.1186/s12935-022-02816-3. Cancer Cell Int. 2022. PMID: 36510217 Free PMC article. Review.
-
PD-1 and PD-L1 Expression in Renal Cell Carcinoma with Sarcomatoid Differentiation.Cancer Immunol Res. 2015 Dec;3(12):1303-7. doi: 10.1158/2326-6066.CIR-15-0150. Epub 2015 Aug 25. Cancer Immunol Res. 2015. PMID: 26307625 Free PMC article.
Cited by
-
Autophagy inhibition upregulates CD4+ tumor infiltrating lymphocyte expression via miR-155 regulation and TRAIL activation.Mol Oncol. 2016 Dec;10(10):1516-1531. doi: 10.1016/j.molonc.2016.08.005. Epub 2016 Sep 16. Mol Oncol. 2016. PMID: 27692344 Free PMC article.
-
Targeted alpha therapy using a novel CD70 targeted thorium-227 conjugate in in vitro and in vivo models of renal cell carcinoma.Oncotarget. 2017 Apr 7;8(34):56311-56326. doi: 10.18632/oncotarget.16910. eCollection 2017 Aug 22. Oncotarget. 2017. PMID: 28915592 Free PMC article.
-
Recent Advances in the Medical Treatment of Recurrent or Metastatic Renal Cell Cancer.Drugs. 2017 Jan;77(1):17-28. doi: 10.1007/s40265-016-0665-1. Drugs. 2017. PMID: 27995579 Review.
-
Pembrolizumab: first experience with recurrent primary central nervous system (CNS) tumors.J Neurooncol. 2016 Sep;129(3):453-460. doi: 10.1007/s11060-016-2190-1. Epub 2016 Jul 4. J Neurooncol. 2016. PMID: 27377654
-
The head and neck cancer immune landscape and its immunotherapeutic implications.JCI Insight. 2016 Oct 20;1(17):e89829. doi: 10.1172/jci.insight.89829. JCI Insight. 2016. PMID: 27777979 Free PMC article.
References
-
- Papac RJ. Spontaneous regression of cancer. Cancer Treat Rev. 1996;22(6):395–423. - PubMed
-
- Nagorsen D, Scheibenbogen C, Marincola FM, Letsch A, Keilholz U. Natural T cell immunity against cancer. Clin Cancer Res. 2003;9(12):4296–4303. - PubMed
-
- Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. - PubMed
-
- Amato RJ, Hawkins RE, Kaufman HL, et al. Vaccination of metastatic renal cancer patients with MVA–5T4: a randomized, double-blind, placebo-controlled phase III study. Clin Cancer Res. 2010;16(22):5539–5547. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

