Chemotherapeutic drugs induce ATP release via caspase-gated pannexin-1 channels and a caspase/pannexin-1-independent mechanism
- PMID: 25112874
- PMCID: PMC4175357
- DOI: 10.1074/jbc.M114.590240
Chemotherapeutic drugs induce ATP release via caspase-gated pannexin-1 channels and a caspase/pannexin-1-independent mechanism
Abstract
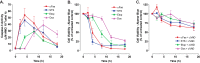
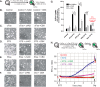
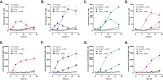
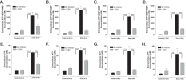
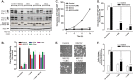
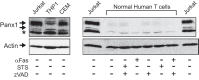
Anti-tumor immune responses have been linked to the regulated release of ATP from apoptotic cancer cells to engage P2 purinergic receptor signaling cascades in nearby leukocytes. We used the Jurkat T cell acute lymphocytic leukemia model to characterize the role of pannexin-1 (Panx1) channels in the release of nucleotides during chemotherapeutic drug-induced apoptosis. Diverse pro-apoptotic drugs, including topoisomerase II inhibitors, kinase inhibitors, and proteosome inhibitors, induced functional activation of Panx1 channels via caspase-3-mediated cleavage of the Panx1 autoinhibitory C-terminal domain. The caspase-activated Panx1 channels mediated efflux of ATP, but also ADP and AMP, with the latter two comprising >90% of the released adenine nucleotide pool as cells transitioned from the early to late stages of apoptosis. Chemotherapeutic drugs also activated an alternative caspase- and Panx1-independent pathway for ATP release from Jurkat cells in the presence of benzyloxycarbonyl-VAD, a pan-caspase inhibitor. Comparison of Panx1 levels indicated much higher expression in leukemic T lymphocytes than in normal, untransformed T lymphoblasts. This suggests that signaling roles for Panx1 may be amplified in leukemic leukocytes. Together, these results identify chemotherapy-activated pannexin-1 channels and ATP release as possible mediators of paracrine interaction between dying tumor cells and the effector leukocytes that mediate immunogenic anti-tumor responses.
Keywords: ATP; Apoptosis; Caspase; Pannexin; Purinergic Agonist.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




Similar articles
-
Up-regulated Ectonucleotidases in Fas-Associated Death Domain Protein- and Receptor-Interacting Protein Kinase 1-Deficient Jurkat Leukemia Cells Counteract Extracellular ATP/AMP Accumulation via Pannexin-1 Channels during Chemotherapeutic Drug-Induced Apoptosis.Mol Pharmacol. 2017 Jul;92(1):30-47. doi: 10.1124/mol.116.104000. Epub 2017 May 1. Mol Pharmacol. 2017. PMID: 28461585 Free PMC article.
-
Pannexin 1 channels mediate 'find-me' signal release and membrane permeability during apoptosis.Nature. 2010 Oct 14;467(7317):863-7. doi: 10.1038/nature09413. Nature. 2010. PMID: 20944749 Free PMC article.
-
Pannexin1 channels act downstream of P2X 7 receptors in ATP-induced murine T-cell death.Channels (Austin). 2014;8(2):142-56. doi: 10.4161/chan.28122. Epub 2014 Mar 3. Channels (Austin). 2014. PMID: 24590064 Free PMC article.
-
Intrinsic properties and regulation of Pannexin 1 channel.Channels (Austin). 2014;8(2):103-9. doi: 10.4161/chan.27545. Epub 2014 Jan 13. Channels (Austin). 2014. PMID: 24419036 Free PMC article. Review.
-
Regulation of Pannexin-1 channel activity.Biochem Soc Trans. 2015 Jun;43(3):502-7. doi: 10.1042/BST20150042. Biochem Soc Trans. 2015. PMID: 26009198 Review.
Cited by
-
Self-Regulation of Memory CD8 T Cell Metabolism through Extracellular ATP Signaling.Immunometabolism. 2019;1(1):e190009. doi: 10.20900/immunometab20190009. Epub 2019 Jul 23. Immunometabolism. 2019. PMID: 31428464 Free PMC article.
-
Caspase-8 as an Effector and Regulator of NLRP3 Inflammasome Signaling.J Biol Chem. 2015 Aug 14;290(33):20167-84. doi: 10.1074/jbc.M115.652321. Epub 2015 Jun 22. J Biol Chem. 2015. PMID: 26100631 Free PMC article.
-
Successful Partnerships: Exploring the Potential of Immunogenic Signals Triggered by TMZ, CX-4945, and Combined Treatment in GL261 Glioblastoma Cells.Int J Mol Sci. 2021 Mar 26;22(7):3453. doi: 10.3390/ijms22073453. Int J Mol Sci. 2021. PMID: 33810611 Free PMC article.
-
Consensus guidelines for the definition, detection and interpretation of immunogenic cell death.J Immunother Cancer. 2020 Mar;8(1):e000337. doi: 10.1136/jitc-2019-000337. J Immunother Cancer. 2020. PMID: 32209603 Free PMC article. Review.
-
Pannexin1 Channels Are Required for Chemokine-Mediated Migration of CD4+ T Lymphocytes: Role in Inflammation and Experimental Autoimmune Encephalomyelitis.J Immunol. 2016 May 15;196(10):4338-47. doi: 10.4049/jimmunol.1502440. Epub 2016 Apr 13. J Immunol. 2016. PMID: 27076682 Free PMC article.
References
-
- Ghiringhelli F., Apetoh L., Tesniere A., Aymeric L., Ma Y., Ortiz C., Vermaelen K., Panaretakis T., Mignot G., Ullrich E., Perfettini J. L., Schlemmer F., Tasdemir E., Uhl M., Génin P., Civas A., Ryffel B., Kanellopoulos J., Tschopp J., André F., Lidereau R., McLaughlin N. M., Haynes N. M., Smyth M. J., Kroemer G., Zitvogel L. (2009) Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β-dependent adaptive immunity against tumors. Nat. Med. 15, 1170–1178 - PubMed
-
- Kepp O., Tesniere A., Zitvogel L., Kroemer G. (2009) The immunogenicity of tumor cell death. Curr. Opin. Oncol. 21, 71–76 - PubMed
-
- Zitvogel L., Kepp O., Galluzzi L., Kroemer G. (2012) Inflammasomes in carcinogenesis and anticancer immune responses. Nat. Immunol. 13, 343–351 - PubMed
-
- Martins I., Michaud M., Sukkurwala A. Q., Adjemian S., Ma Y., Shen S., Kepp O., Menger L., Vacchelli E., Galluzzi L., Zitvogel L., Kroemer G. (2012) Premortem autophagy determines the immunogenicity of chemotherapy-induced cancer cell death. Autophagy 8, 413–415 - PubMed
-
- Michaud M., Martins I., Sukkurwala A. Q., Adjemian S., Ma Y., Pellegatti P., Shen S., Kepp O., Scoazec M., Mignot G., Rello-Varona S., Tailler M., Menger L., Vacchelli E., Galluzzi L., Ghiringhelli F., di Virgilio F., Zitvogel L., Kroemer G. (2011) Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 334, 1573–1577 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

