The crucial role of Atg5 in cortical neurogenesis during early brain development
- PMID: 25109817
- PMCID: PMC4127499
- DOI: 10.1038/srep06010
The crucial role of Atg5 in cortical neurogenesis during early brain development
Abstract
Autophagy plays an important role in the central nervous system. However, it is unknown how autophagy regulates cortical neurogenesis during early brain development. Here, we report that autophagy-related gene 5 (Atg5) expression increased with cortical development and differentiation. The suppression of Atg5 expression by knockdown led to inhibited differentiation and increased proliferation of cortical neural progenitor cells (NPCs). Additionally, Atg5 suppression impaired cortical neuronal cell morphology. We lastly observed that Atg5 was involved in the regulation of the β-Catenin signaling pathway. The β-Catenin phosphorylation level decreased when Atg5 was blocked. Atg5 cooperated with β-Catenin to modulate cortical NPCs differentiation and proliferation. Our results revealed that Atg5 has a crucial role in cortical neurogenesis during early embryonic brain development, which may contribute to the understanding of neurodevelopmental disorders caused by autophagy dysregulation.
Figures
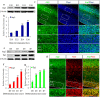
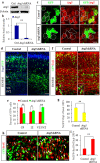
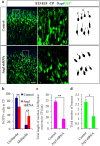
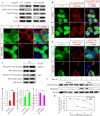
Similar articles
-
Atg5 and Ambra1 differentially modulate neurogenesis in neural stem cells.Autophagy. 2012 Feb 1;8(2):187-99. doi: 10.4161/auto.8.2.18535. Epub 2012 Feb 1. Autophagy. 2012. PMID: 22240590
-
Wnt signaling regulates neuronal differentiation of cortical intermediate progenitors.J Neurosci. 2011 Feb 2;31(5):1676-87. doi: 10.1523/JNEUROSCI.5404-10.2011. J Neurosci. 2011. PMID: 21289176 Free PMC article.
-
Knockout of Atg5 delays the maturation and reduces the survival of adult-generated neurons in the hippocampus.Cell Death Dis. 2016 Mar 3;7(3):e2127. doi: 10.1038/cddis.2015.406. Cell Death Dis. 2016. PMID: 26938300 Free PMC article.
-
Wnt/β-Catenin Signaling Pathway Governs a Full Program for Dopaminergic Neuron Survival, Neurorescue and Regeneration in the MPTP Mouse Model of Parkinson's Disease.Int J Mol Sci. 2018 Nov 24;19(12):3743. doi: 10.3390/ijms19123743. Int J Mol Sci. 2018. PMID: 30477246 Free PMC article. Review.
-
Evaluation of advances in cortical development using model systems.Dev Neurobiol. 2022 Jul;82(5):408-427. doi: 10.1002/dneu.22879. Epub 2022 May 29. Dev Neurobiol. 2022. PMID: 35644985 Free PMC article. Review.
Cited by
-
TBR2 coordinates neurogenesis expansion and precise microcircuit organization via Protocadherin 19 in the mammalian cortex.Nat Commun. 2019 Sep 2;10(1):3946. doi: 10.1038/s41467-019-11854-x. Nat Commun. 2019. PMID: 31477701 Free PMC article.
-
FoxO Function Is Essential for Maintenance of Autophagic Flux and Neuronal Morphogenesis in Adult Neurogenesis.Neuron. 2018 Sep 19;99(6):1188-1203.e6. doi: 10.1016/j.neuron.2018.08.017. Epub 2018 Sep 6. Neuron. 2018. PMID: 30197237 Free PMC article.
-
Enhancing Lysosomal Activation Restores Neural Stem Cell Function During Aging.J Exp Neurosci. 2018 Aug 23;12:1179069518795874. doi: 10.1177/1179069518795874. eCollection 2018. J Exp Neurosci. 2018. PMID: 30158826 Free PMC article.
-
Autophagy in major human diseases.EMBO J. 2021 Oct 1;40(19):e108863. doi: 10.15252/embj.2021108863. Epub 2021 Aug 30. EMBO J. 2021. PMID: 34459017 Free PMC article. Review.
-
Ependymal and Neural Stem Cells of Adult Molly Fish (Poecilia sphenops, Valenciennes, 1846) Brain: Histomorphometry, Immunohistochemical, and Ultrastructural Studies.Cells. 2022 Aug 27;11(17):2659. doi: 10.3390/cells11172659. Cells. 2022. PMID: 36078068 Free PMC article.
References
-
- Angevine J. B. Jr & Sidman R. L. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature 192, 766–768 (1961). - PubMed
-
- Temple S. & Qian X. Vertebrate neural progenitor cells: subtypes and regulation. Curr Opin Neurobiol 6, 11–17 (1996). - PubMed
-
- Xie Z. & Klionsky D. J. Autophagosome formation: core machinery and adaptations. Nat Cell Biol 9, 1102–1109 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

