A highly specific monoclonal antibody against monkeypox virus detects the heparin binding domain of A27
- PMID: 25108113
- PMCID: PMC9629035
- DOI: 10.1016/j.virol.2014.06.039
A highly specific monoclonal antibody against monkeypox virus detects the heparin binding domain of A27
Abstract
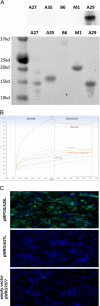
The eradication of smallpox and the cessation of global vaccination led to the increased prevalence of human infections in Central Africa. Serologic and protein-based diagnostic assay for MPXV detection is difficult due to cross-reactive antibodies that do not differentiate between diverse orthopoxvirus (OPXV) species. A previously characterized monoclonal antibody (mAb 69-126-3-7) against MPXV [1] was retested for cross-reactivity with various OPXVs. The 14.5 kDa band protein that reacted with mAb 69-126-3 was identified to be MPXV A29 protein (homolog of vaccinia virus Copenhagen A27). Amino acid sequence analysis of the MPXV A29 with other OPXV homologs identified four amino acid changes. Peptides corresponding to these regions were designed and evaluated for binding to mAb 69-126-3 by ELISA and BioLayer Interferometry (BLI). Further refinement and truncations mapped the specificity of this antibody to a single amino acid difference in a 30-mer peptide compared to other OPXV homologs. This particular residue is proposed to be essential for heparin binding by VACV A27 protein. Despite this substitution, MPXV A29 bound to heparin with similar affinity to that of VACV A27 protein, suggesting flexibility of this motif for heparin binding. Although binding of mAb 69-126-3-7 to MPXV A29 prevented interaction with heparin, it did not have any effect on the infectivity of MPXV. Characterization of 69-126-3-7 mAb antibody allows for the possibility of the generation of a serological based species-specific detection of OPXVs despite high proteomic homology.
Keywords: Antibody; Heparin binding; Monkeypox virus; Orthopoxvirus; Vaccinia virus.
Published by Elsevier Inc.
Figures




Similar articles
-
Monkeypox virus A29L protein as the target for specific diagnosis and serological analysis.Appl Microbiol Biotechnol. 2024 Nov 21;108(1):522. doi: 10.1007/s00253-024-13361-6. Appl Microbiol Biotechnol. 2024. PMID: 39570405 Free PMC article.
-
Unexpectedly higher levels of anti-orthopoxvirus neutralizing antibodies are observed among gay men than general adult population.BMC Med. 2023 May 16;21(1):183. doi: 10.1186/s12916-023-02872-0. BMC Med. 2023. PMID: 37189197 Free PMC article.
-
Linear Epitopes in Vaccinia Virus A27 Are Targets of Protective Antibodies Induced by Vaccination against Smallpox.J Virol. 2016 Apr 14;90(9):4334-4345. doi: 10.1128/JVI.02878-15. Print 2016 May. J Virol. 2016. PMID: 26889021 Free PMC article.
-
Monkeypox virus: a re-emergent threat to humans.Virol Sin. 2022 Aug;37(4):477-482. doi: 10.1016/j.virs.2022.07.006. Epub 2022 Jul 9. Virol Sin. 2022. PMID: 35820590 Free PMC article. Review.
-
Monkeypox virus and insights into its immunomodulatory proteins.Immunol Rev. 2008 Oct;225:96-113. doi: 10.1111/j.1600-065X.2008.00691.x. Immunol Rev. 2008. PMID: 18837778 Free PMC article. Review.
Cited by
-
Design and Optimization of a Monkeypox virus Specific Serological Assay.Pathogens. 2023 Mar 1;12(3):396. doi: 10.3390/pathogens12030396. Pathogens. 2023. PMID: 36986317 Free PMC article.
-
Kinetic and Structural Aspects of Glycosaminoglycan-Monkeypox Virus Protein A29 Interactions Using Surface Plasmon Resonance.Molecules. 2022 Sep 11;27(18):5898. doi: 10.3390/molecules27185898. Molecules. 2022. PMID: 36144634 Free PMC article.
-
Monkeypox epidemic at the door: should we remain idly by or prepare strongly?AMB Express. 2023 Jan 13;13(1):5. doi: 10.1186/s13568-023-01507-0. AMB Express. 2023. PMID: 36637577 Free PMC article. Review.
-
Biological Characteristics and Pathogenesis of Monkeypox Virus: An Overview.Adv Exp Med Biol. 2024;1451:91-109. doi: 10.1007/978-3-031-57165-7_6. Adv Exp Med Biol. 2024. PMID: 38801573 Review.
-
Electrochemical Immunoassay Platform for Human Monkeypox Virus Detection.Anal Chem. 2024 May 28;96(21):8342-8348. doi: 10.1021/acs.analchem.3c05182. Epub 2024 May 10. Anal Chem. 2024. PMID: 38728056 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

